A lanthanide metal–organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants†
Abstract
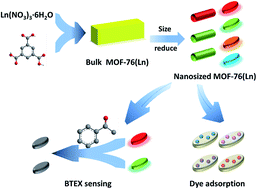
A series of nano-sized lanthanide metal–organic frameworks (Ln-MOFs) which are isostructural with parent MOF-76(Ln) (Ln = Eu, Tb, Sm, Dy) are synthesized under solvothermal conditions. The morphology and particle size of the Ln-MOFs demonstrated by scanning electron microscopy can be changed by increasing the quantities of sodium acetate whose capping effect provides more coordinate sites for lanthanide ions. Furthermore, we explore the adsorption properties of nanosized MOF-76(Ln) for organic dyes, indicating that nanosized MOF-76(Ln) can adsorb some cationic dyes like methylene blue, etc. In addition, MOF-76(Eu) and MOF-76(Tb) are selected for fluorescence sensing for monoaromatic hydrocarbons (BTEX). Such system serves as an acetophenone detection platform through a fluorescence quench process. This work highlights the practical application of MOF-76(Ln) as sensors, and it paves the way for detecting BTEX in solution or in vapor phase.
- This article is part of the themed collection: Zeolites and 3D Porous Solids

 Please wait while we load your content...
Please wait while we load your content...