One-step synthesis of magnetic iron–aluminum oxide/graphene oxide nanoparticles as a selective adsorbent for fluoride removal from aqueous solution†
Lei Liu,
Zhaojie Cui*,
Qianchi Ma,
Wei Cui and
Xu Zhang
School of Environmental Science and Engineering, Shandong University, 27 Shanda Nanlu, Ji’nan, 250100, China. E-mail: cuizj@sdu.edu.cn; Fax: +86 531 88361176; Tel: +86 531 88361176
First published on 15th January 2016
Abstract
A novel magnetic adsorbent consisting of iron–aluminum oxide nanoparticles anchored on graphene oxide (IAO/GO) was prepared through a simple one-step co-precipitation method for fluoride removal from aqueous solution. Through this one-step method, this study simplified the operation processes to realize the magnetic composite of a binary iron–aluminum mixed oxide and graphene oxide (GO). By combining the advantages of GO and IAO, IAO/GO exhibits high adsorption capacity, good acid–alkali stability, super paramagnetism and good selectivity for fluoride. With magnetic properties, the adsorbent could easily be collected from aqueous solution using an external magnetic field. The physicochemical properties of IAO/GO were characterized through N2 adsorption/desorption, XRD, TEM, XPS, FT-IR, and AGM. Several main factors, such as dosage, initial solution pH, contact time, initial fluoride concentration, and co-existing anions, were investigated. Kinetic data revealed that the adsorption process followed a pseudo-second-order model. Fluoride sorption onto the adsorbents fitted well with the Langmuir model. The maximum sorption capacity calculated from the Langmuir model was 64.72 mg g−1 for IAO/GO. Effective fluoride removal occurred in a wide pH range from 3 to 9. IAO/GO showed good selectivity for fluoride when anions existed except for HPO42−. According to the sorption studies, electrostatic attraction, anion exchange, and inner-sphere complexation were the most likely mechanisms for fluoride sorption. Overall, based on the above-mentioned merits, the IAO/GO prepared in this study could be applied widely for fluoride removal in natural water environments.
1. Introduction
Fluoride is an essential trace element in humans.1 However, excessive fluoride intake can cause severe health problems, such as dental and skeletal fluorosis.2 Fluoride is mainly released from the slow dissolution of fluorine-containing rocks and is generated by human activities such as the glass industry, chemical industry and metal industry.3,4 According to the World Health Organization, fluoride intake should be lower than 1.5 mg L−1.5 Unfortunately, over 200 million people suffer from elevated fluoride levels in drinking water worldwide.6,7 In China, a population of 45 million in more than 30 provinces is affected by endemic fluorosis.8 Thus, a stable and effective method for the removal of fluoride in drinking water is urgently needed.Many studies have been conducted on the elimination of fluoride in drinking water.9–12 Most of these studies focused on the adsorption method because it is simple and cost effective.9 Various adsorbents have been reported for fluoride removal, such as activated and impregnated alumina,13 alum sludge,14 iron oxide,1 mixed metal oxides,15,16 layered double hydroxides,17 and carbonaceous material impregnated with rare earth metals.12,18 Among these adsorbents, iron and aluminum oxides have received the most attention in previous studies because of their low cost and high affinity toward fluoride.19–21
Recently, magnetic adsorbents have attracted great attention over these adsorbents because of their easy separation after treatment. With magnetic properties, the quick separation of solid–liquid phases can be realized with an external magnetic field. Some magnetic adsorbents have been reported by other researches, such as hydrous aluminum oxide embedded with Fe3O4,22 sulfate-doped Fe3O4/Al2O3 nanoparticles,6 a magnetic Fe3O4/MgAl-LDH composite23 and a polypyrrole/Fe3O4 magnetic nanocomposite.24 But these adsorbents were synthesized using a multi-step method to obtain a core–shell structure. Fe3O4 was synthesized firstly, and then performed as the core of the adsorbent to realize the magnetic properties. Actually, iron oxide also has a good affinity toward fluoride,1 but the core–shell structure prohibited the adsorption ability of the iron oxide. Our aim is to simplify the preparation process through using a one-step method to obtain a binary iron–aluminum mixed oxide which possesses magnetic properties and a high capacity.
Graphene oxide (GO), a desirable adsorbent material, consists of abundant oxygen-containing functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, –OH)25 and has a large surface area. But its good hydrophilicity limits direct application due to the difficulty of collecting GO from solution.26 From another point of view, GO can serve as a promising support material to load nanoparticles.27 Many researchers28–30 have reported the introduction of GO into photocatalytic and adsorbent materials, but most of these GO supported materials were loaded with only a single metal oxide.
O, C–O, –OH)25 and has a large surface area. But its good hydrophilicity limits direct application due to the difficulty of collecting GO from solution.26 From another point of view, GO can serve as a promising support material to load nanoparticles.27 Many researchers28–30 have reported the introduction of GO into photocatalytic and adsorbent materials, but most of these GO supported materials were loaded with only a single metal oxide.
In this present study, to combine the advantages of GO and binary iron–aluminum oxide (IAO) and to realize a quick collection, a one-step co-precipitation method31 was used to load the IAO onto the surface of the GO and achieve magnetic properties. The physiochemical characteristics were evaluated through N2 adsorption/desorption, X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Fourier transformed infrared spectroscopy (FT-IR) and using an alternating gradient magnetometer (AGM). The mechanisms of fluoride adsorption onto IAO/GO were comprehensively investigated through batch experiments (equilibration time, pH, fluoride concentration, and co-existing anions) and instrumental analyses. Our study demonstrates that IAO/GO can be an effective adsorbent for fluoride removal and separates it fast from aqueous solution.
2. Materials and methods
2.1 Materials
All of the chemicals were of analytical grade and used without further treatment. Graphite powder (300 mesh) was purchased from Guangnuo Chemical Technology Co., Ltd. (Shanghai, China). Aluminum chloride (AlCl3·6H2O), ferric chloride (FeCl3·6H2O), and ferrous chloride (FeCl2·4H2O) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The stock solution of fluoride (1000 mg F− per L) was prepared using deionized water and sodium fluoride (NaF), stored in cold storage, and diluted when ready for use.2.2 Adsorbent preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2+
Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ = 1.5
Al3+ = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5) were sequentially added to the GO solution. After the solution was mixed uniformly, ammonia solution (28%) was slowly added into the above solution under vigorous mechanical stirring until the pH of the solution reached 10 at room temperature. The temperature was raised to 65 °C and stirred for 1 h under a N2 atmosphere. The solution was cooled to room temperature and further aged for 24 h. The precipitate was collected by filtration, washed with deionized water and ethanol until it was neutral pH, and dried under vacuum at 60 °C for 10 h. Finally, the precipitate was ground to obtain a particle size of 100–160 mesh with standard sieves (Model Φ200), calcined under a N2 (100 mL min−1) atmosphere at 200 °C with a heating rate of 10 °C min−1 for 2 h in a tube furnace, cooled to room temperature, and stored until required.
4.5) were sequentially added to the GO solution. After the solution was mixed uniformly, ammonia solution (28%) was slowly added into the above solution under vigorous mechanical stirring until the pH of the solution reached 10 at room temperature. The temperature was raised to 65 °C and stirred for 1 h under a N2 atmosphere. The solution was cooled to room temperature and further aged for 24 h. The precipitate was collected by filtration, washed with deionized water and ethanol until it was neutral pH, and dried under vacuum at 60 °C for 10 h. Finally, the precipitate was ground to obtain a particle size of 100–160 mesh with standard sieves (Model Φ200), calcined under a N2 (100 mL min−1) atmosphere at 200 °C with a heating rate of 10 °C min−1 for 2 h in a tube furnace, cooled to room temperature, and stored until required.For comparison, bare IAO nanoparticles were prepared following the aforementioned procedures, except for the addition of GO.
2.3 Characterization
The specific surface area was determined using the Brunauer–Emmett–Teller (BET) method with a surface area analyzer (SI/MP, Quantachrome, USA). Powder X-ray diffraction (XRD, Rigaku D/MAX-rA, Japan) was performed with Cu Kα radiation (λ = 1.5418 Å), an accelerating voltage of 40 kV, and a current of 40 mA to analyze the structure and mineralogy of the adsorbents. The morphology of GO, IAO, and IAO/GO were determined through transmission electron microscopy (TEM, JEM-2100F, JEOL, Japan). The elemental composition of IAO/GO was determined semi-quantitatively using energy-dispersive X-ray spectrometry. The surface of the IAO/GO before and after fluoride adsorption was recorded using X-ray photoelectron spectroscopy (XPS, ESCALAB 250, ThermoFisher SCIENTIFIC) with monochromatic Al Kα radiation (1486.6 eV). The C 1s peak (284.6 eV) was used to calibrate all binding energies. The Fourier transformed infrared spectroscopy (FT-IR) spectra of GO, IAO, and IAO/GO were recorded from 400 cm−1 to 4000 cm−1 using a Nicolet 380 spectrometer (Thermo Fisher Scientific, USA). The magnetic properties were measured with an alternating gradient magnetometer (AGM, 2900-04C, PMC, USA) at room temperature.2.4 Adsorption experiments
Batch adsorption experiments were conducted to study the effect of adsorbent dosage, equilibration time, pH, fluoride concentration, and co-existing anions. All of the experiments were performed in 100 mL polyethylene vials filled with 100 mL of the fluoride solution and shaken at 200 rpm and 25 ± 1 °C for 20 h. The samples were filtered using a 0.45 μm membrane filter. The fluoride concentration in the solutions was measured using the fluoride selective electrode method according to the Chinese standard GB 7484-87 (water quality-determination of fluoride-ion selective electrode method) with a digital ion meter (Model PXS-215, Shanghai, China).In the dosage experiments, a 0.01–0.1 g dosage was added to 100 mL of the fluoride solution (10 mg L−1 and pH: 6.5 ± 0.2). For the fluoride adsorption isotherm, the initial fluoride concentration was varied from 2 mg L−1 to 50 mg L−1 with a dosage of 0.025 g/100 mL at pH 6.5 ± 0.2. For the kinetic analysis, a series of vials were prepared with the same conditions (initial fluoride concentration = 10 mg L−1 and adsorbent dosage = 0.025 g/100 mL). One of the vials was filtered immediately to determine the fluoride concentration after adsorption at a certain time interval. The experiment of the initial pH effect on fluoride removal was conducted by adjusting the pH from 2.0 to 11.0 using 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH (initial fluoride concentration = 10 mg L−1 and dosage = 0.025 g/100 mL). The final pH was recorded with a digital ion meter (Model PXS-215, Shanghai, China). The levels of iron and aluminum ions released from the adsorbent at different initial pH values were simultaneously determined using inductively coupled plasma-atomic emission spectrometry (IRIS Intrepid II, Thermo Electron Corporation, USA). Meanwhile, control samples without fluoride were prepared to record the changes in pH, as well as the levels of iron and aluminum ions. The pH at the point of zero charge (pHPZC) of IAO/GO and IAO was also determined according to the control samples through a batch equilibrium method described by Babić.33 The influences of co-existing anions (nitrate, chloride, sulfate, and hydrogen phosphate) on fluoride adsorption were tested under a fixed initial fluoride concentration (10 mg L−1) and the initial co-existing anion concentrations of 10 and 100 mmol L−1 at pH 6.5 ± 0.2. The adsorbent-phase concentration of the adsorbate (qt, mg g−1) at any time t was calculated using eqn (1) as follows:
3. Results and discussion
3.1 Characterization of the composites
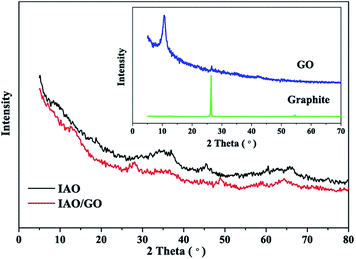
The results of the BET analysis showed that the specific surface areas were 303 and 349 m2 g−1 for IAO and IAO/GO, respectively. The combination of GO and IAO resulted in an orderly arrangement of the IAO particles, which were anchored on the GO layers, thereby hindering the IAO particles from agglomeration.34 Thus, the specific surface area of IAO/GO showed a slight increase compared with that of IAO.Fig. 1 shows the XRD patterns of graphite, GO, IAO, and IAO/GO. Graphite (inset) exhibited a sharp peak (002) at 2θ = 26.41°, indicating the interlayer spacing of 0.337 nm. GO (inset) displayed a new peak (001) at 2θ = 10.85° with a 0.81 nm d-spacing after the oxidation of graphite.34 The increased d-spacing is due to the formation of oxygen-containing functional groups during the oxidation of graphite. The introduction of these groups resulted in the loose stacking of GO sheets and weakened π–π stacking.35 IAO and IAO/GO did not show obvious diffraction peaks, which indicated that both IAO and IAO/GO did not have a fine crystalline structure. This situation might be attributed to the low calcination temperature (200 °C),16 and the co-precipitation process of Al3+ and Fe3+/Fe2+ could disrupt crystal formation.36,37 Interestingly, the characteristic peak of GO at 2θ = 10.85° disappeared in the IAO/GO composite. This phenomenon can be explained by the conclusion of a previous study,38 which reported that regular stacks of GO are destroyed by exfoliation and sonication during adsorbent preparation, so the diffraction peaks become weak or disappear. In the synthesis of the composite, the particles anchored on the surface of the GO sheets prevented the restacking of the sheets, so the characteristic peak disappeared.
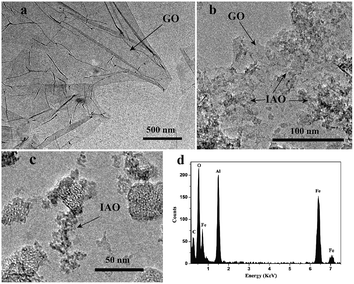
The TEM images of GO, IAO, and IAO/GO are shown in Fig. 2. Fig. 2a reveals that the GO sheets exhibits a typically wrinkled structure.39 Corresponding with the XRD patterns, Fig. 2b illustrates that IAO was distributed onto the GO sheets with an amorphous structure. Compared with the large aggregated IAO particles shown in Fig. 2c, IAO was distributed homogeneously onto the GO layers. Fig. 2d shows that the IAO/GO consisted of C, O, Al, and Fe, which indicated that the GO sheets were coated with IAO.
The XPS survey spectra of IAO/GO before and after reaction with fluoride are shown in Fig. 3. The XPS survey spectrum indicates that the sample contained C, O, Al, and Fe with peaks at the binding energies of 284.5 (C 1s), 530.9 (O 1s), 73.5 (Al 2p), and 711.8 (Fe 2p). The F 1s peak was observed at 685.1 eV after fluoride adsorption, which indicates the successful bonding of fluoride to the adsorbent.
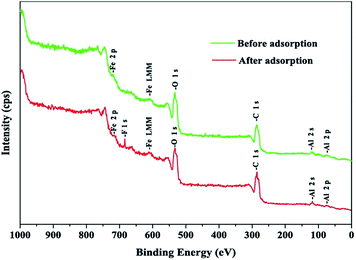 | ||
| Fig. 3 The X-ray photoelectron spectroscopy (XPS) survey spectra of IAO/GO before and after fluoride adsorption. | ||
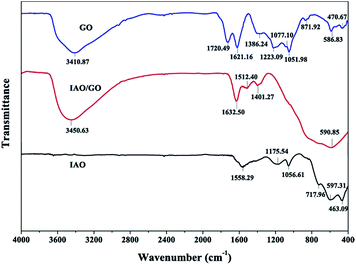
The FT-IR spectra of GO, IAO, and IAO/GO are presented in Fig. 4. For the GO curve, the peaks at 3411 and 1223 cm−1 indicate the O–H stretching and bending vibrations, respectively. The peak at 1720 cm−1 was attributed to the stretching band of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic acid or carbonyl moieties. The peaks at 1386 and 1077 cm−1 correspond to other oxygen-containing functional groups (epoxide and alkoxy). The peak at 1052 cm−1 revealed the deformation of C–O. The band of the skeletal vibrations of unoxidized graphitic domains appears at 1621 cm−1.27,40 All of the above peaks suggested that GO was prepared successfully. For the IAO curve, the absorption bands in the range of 500–800 cm−1 were assigned to the M–O, O–M–O, and M–O–M vibrations (M = Fe, Al).41,42 The band at 1056 cm−1 could be attributed to the bending vibration of the metal oxide hydroxyl groups (MOH).43 This finding showed the formation of IAO. By contrast, the FT-IR spectrum of IAO/GO differed from that of GO or IAO possibly because the combination of GO and IAO may strengthen or weaken the original respective characteristic peaks. The absorption band at 3450 cm−1 could be considered a new peak compared with IAO, this band could be attributed to the O–H stretching of GO. The peaks between 500–800 cm−1 were strengthened compared with the GO spectrum. All of these facts indicate that the composite of IAO/GO was synthesized successfully.
O in carboxylic acid or carbonyl moieties. The peaks at 1386 and 1077 cm−1 correspond to other oxygen-containing functional groups (epoxide and alkoxy). The peak at 1052 cm−1 revealed the deformation of C–O. The band of the skeletal vibrations of unoxidized graphitic domains appears at 1621 cm−1.27,40 All of the above peaks suggested that GO was prepared successfully. For the IAO curve, the absorption bands in the range of 500–800 cm−1 were assigned to the M–O, O–M–O, and M–O–M vibrations (M = Fe, Al).41,42 The band at 1056 cm−1 could be attributed to the bending vibration of the metal oxide hydroxyl groups (MOH).43 This finding showed the formation of IAO. By contrast, the FT-IR spectrum of IAO/GO differed from that of GO or IAO possibly because the combination of GO and IAO may strengthen or weaken the original respective characteristic peaks. The absorption band at 3450 cm−1 could be considered a new peak compared with IAO, this band could be attributed to the O–H stretching of GO. The peaks between 500–800 cm−1 were strengthened compared with the GO spectrum. All of these facts indicate that the composite of IAO/GO was synthesized successfully.
The magnetization hysteresis loops of Fe3O4, IAO, and IAO/GO at room temperature were measured using an AGM and are shown in Fig. 5. The saturation magnetization value of Fe3O4 is 60 emu g−1. However, the saturation magnetization values of IAO and IAO/GO dramatically decreased to 10 and 7.5 emu g−1, respectively. This decrease could be ascribed to the low content of iron oxide in the adsorbents and the introduction of aluminum oxide, which may affect the crystallization of iron oxide.22,36 Fortunately, both of the adsorbents still exhibited excellent typical superparamagnetic behavior, and they could be separated quickly with an external magnetic field.
3.2 Effect of dosage on fluoride removal
The effects of adsorbent dose on the residual fluoride concentration, removal efficiency, and capacity are shown in Fig. 6. The fluoride removal efficiency drastically increased with the IAO/GO dose from 0.1 to 0.6 g L−1, but no significant increase occurred beyond this dose (Fig. 6a). By contrast, the removal efficiency for IAO showed a gradual increase up to 1.0 g L−1. The removal efficiency of fluoride could reach nearly 100% at a dose of 0.6 g L−1 with IAO/GO but only 75% with IAO. The data (Fig. 6b) showed that the residual fluoride concentration could meet the recommended concentration level of 1.5 mg L−1 with 0.25–0.3 g L−1 of IAO/GO. Therefore, 0.25 g L−1 of IAO/GO was optimal for the next adsorption tests.3.3 Kinetic analysis
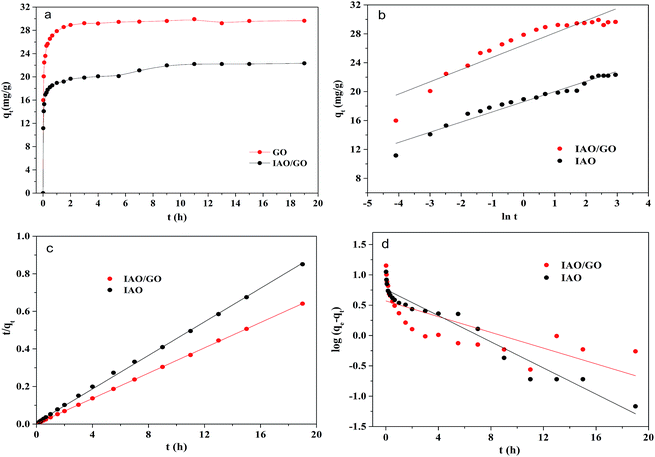
The kinetic results of the fluoride adsorption onto IAO and IAO/GO are shown in Fig. 7a. The two adsorbents both showed rapid uptake during the first 30 min, followed by slower adsorption rates until equilibrium. IAO/GO reached its equilibrium within 3 h, whereas IAO required 11 h. Three kinetic models, the pseudo-first-order model (eqn (2)), pseudo-second-order model (eqn (3)), and the Elovich model (eqn (4)), were used to analyze the adsorption kinetics.Pseudo-first-order model:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (2) |
Pseudo-second-order model:
 | (3) |
Elovich model:
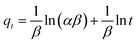 | (4) |
The fitting of the three models to the experimental data is shown in Fig. 7b–d, and the parameters of the three models are summarized in Table 1. The pseudo-second-order model correlated better with the experimental data (R2 = 0.9999 for IAO/GO and 0.9991 for IAO) than the pseudo-first-order and Elovich models. The qe values calculated (qe (cal)) from the pseudo-second-order kinetic equation were in good agreement with the experimental qe (qe (exp)). Therefore, fluoride adsorption onto both IAO and IAO/GO obeyed pseudo-second-order kinetics in the entire process. This indicated that the adsorption process may be dominated by chemical bonding between the active sites and fluoride.
| Samples | qe (exp) (mg g−1) | Pseudo-first-order | Pseudo-second-order | Elovich model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe (cal) (mg g−1) | R2 | k2 (g (mg−1 h−1)) | qe (cal) (mg g−1) | R2 | α | β | R2 | ||
| IAO | 22.33 | 0.108 | 2.138 | 0.9487 | 0.747 | 22.38 | 0.9991 | 7.762 × 105 | 0.711 | 0.9571 |
| IAO/GO | 29.65 | 0.065 | 1.771 | 0.5699 | 0.245 | 29.68 | 0.9999 | 1.019 × 107 | 0.590 | 0.8606 |
3.4 Adsorption isotherm
The adsorption isotherms of fluoride onto IAO and IAO/GO are shown in Fig. 8. The Langmuir (eqn (5)) and Freundlich (eqn (6)) models44 were used to fit the experimental data.
 | (5) |
| qe = KFCe1/n | (6) |
Table 2 lists the parameters of the Langmuir and Freundlich equations. As shown in Fig. 8, the Langmuir model fitted better than the Freundlich model, which involved the monolayer adsorption of fluoride by the adsorbent.15 According to the Langmuir model, the maximum sorption capacity (qm, mg g−1) was calculated as 64.72 mg g−1 for IAO/GO and 46.54 mg g−1 for IAO. The maximum sorption capacity of IAO/GO increased significantly by nearly 40% compared with that of IAO. This increase was probably due to the addition of GO in the preparation process of the adsorbent material. The possible reasons could be as follows: (1) IAO/GO had a higher BET value than IAO, so IAO/GO had more available binding sites for fluoride than IAO; (2) GO possessed some oxygen-containing functional groups, and the introduction of GO to IAO resulted in more oxygen-containing functional groups to the adsorbent.
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | KF (mg1−n Ln g−1) | n | R2 | |
| IAO | 46.54 | 0.256 | 0.9928 | 14.242 | 3.101 | 0.9434 |
| IAO/GO | 64.72 | 0.314 | 0.9956 | 20.991 | 3.121 | 0.9368 |
In addition, adsorbent properties for fluoride removal previously reported in the literature are listed in Table 3. It is obvious that the capacity of IAO/GO is higher under similar experimental conditions compared with most of the other adsorbents.6,15,19,22,24,37,45
| Adsorbent | Superparamagnetism (emu g−1) | pH | qma (mg g−1) | Ref. |
|---|---|---|---|---|
| a Adsorption capacity using Langmuir isothermal fitting. | ||||
| IAO/GO | 7.5 | 6.5 | 64.72 | This study |
| Alum-impregnated activated alumina | — | 6.5 | 40.68 | Tripathy et al., 2006 |
| Iron(III)–aluminum(III) mixed oxide | — | 6.9 | 17.73 | Biswas et al., 2007 |
| Hydrated iron(III)–aluminum(III)–chromium(III) ternary mixed oxide | — | 5.6 | 31.89 | Biswas et al., 2010 |
| Fe3O4@Al(OH)3 | 17.9 | 6.5 | 88.48 | Zhao et al., 2010 |
| Polypyrrole/Fe3O4 | Not given | 6.5 | 17.6–22.3 | Bhaumik et al., 2011 |
| Sulfate-doped Fe3O4/Al2O3 | Not given | 7.0 | 48.5 | Chai et al., 2013 |
| Fe–Ti oxide | — | — | 47.0 | Chen et al., 2012 |
3.5 Effect of pH
The initial pH is a main factor that affects fluoride adsorption.22 The pH values of the samples before and after adsorption are plotted in Fig. 9a. The pHpzc values for IAO/GO and IAO are 6.2 and 7.0 (Fig. 9a, the pHfinal level is where the common plateau is obtained from the control samples), respectively. The final pH of the control samples was lower than that of the samples with fluoride. This result indicates that anion exchange may play a role in fluoride adsorption.46 Moreover, fluoride adsorption is coupled with the release of hydroxyl ions, so low pH is beneficial for fluoride removal. The results of the present study proved the competitive adsorption between fluoride and OH−.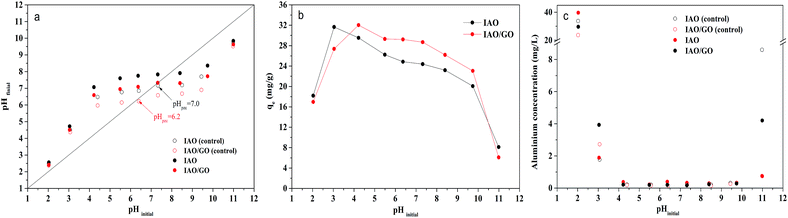 | ||
| Fig. 9 The pH in the solution after equilibrium (a); the adsorption capacity (mg g−1) (b) and aluminum concentration in solution (c) as a function of the initial pH. | ||
Fig. 9b displays the fluoride adsorption capacity (qe, mg g−1) of IAO and IAO/GO with pH ranging from 2.0 to 11.0. The maximum adsorption capacity for IAO (31.6 mg g−1) and IAO/GO (32.0 mg g−1) occurred at the initial pH of 3.04 and 4.22, respectively. At pH of 4.0–10.0, IAO/GO exhibited a higher adsorption capacity than IAO, but the capacities of IAO/GO and IAO both slightly decreased with increasing pH. Besides, IAO/GO and IAO showed a dramatic decline in adsorption capacity at pH < 3.0 and pH > 10.0, respectively. The above phenomenon may be due to several reasons: at pH < 3.0, fluoride ions are transformed into electrically neutral hydrofluoric acid, which can diminish the electrostatic attraction between the adsorbent surface and fluoride,6 and the adsorbent may exhibit a slight loss of solubility due to the strong acid. At pH > 10.0, the adsorbent surface was strongly negative, which repelled the fluoride ions through electrostatic repulsion, and the high concentration of hydroxyl ions could compete with the fluoride ions to occupy the adsorption sites.6,22 The adsorbent may also show a loss of solubility, as evidenced by the formation of Al(OH)4− resulting from the dissolution of alumina in the high pH solution. Above pHpzc, the adsorption capacity did not decline dramatically to a very low value, which revealed that electrostatic adsorption was not the major adsorption mechanism.
The residual aluminum and iron concentrations of the solutions after filtration were examined, and the data are plotted in Fig. 9c. Iron was not detected, except at pH 2 (data not shown). The aluminum concentrations were 0.1–0.2 mg L−1 at pH 4–10, which are lower than the value (0.2 mg L−1) proposed by the World Health Organization, but it was much higher at pH 2 and 11. These findings demonstrated the loss in solubility of the adsorbent in strong acid and alkali environments. Considering the high capacity and low solubility, IAO/GO can be applied in a wide pH range.
3.6 Effects of coexisting anions
The effects of co-existing anions at different concentrations (10 and 100 mmol L−1) on fluoride removal are presented in Fig. 10. Nitrate and chloride had minimal effect on sorption, whereas hydrogen phosphate demonstrated the greatest competitive influence among the anions. Their interference followed the decreasing order of HPO42− > SO42− > Cl− > NO3−, and this order closely correlates with the order of the charge/radius values of the anions.22 Chloride and nitrate form outer-sphere complexes, hydrogen phosphate forms inner-sphere complexes, and sulfate can partially form outer-sphere complexes or inner-sphere complexes.47,48 Based on the decreasing order of the anions, the fluoride adsorbed via inner-sphere complexation, because hydrogen phosphate competed with fluoride for the same active sites by forming inner-sphere complexes.4. Conclusions
IAO/GO, a novel adsorbent was successfully synthesized using a one-step co-precipitation method, which realized the loading of binary iron–aluminum oxide onto the surface of GO and achieved a magnetic performance, simultaneously. IAO/GO exhibited higher adsorption capacity (64.72 mg g−1) toward fluoride than some other reported materials and can be collected quickly from solution with an external magnet. Kinetic studies indicated that the adsorption process obeyed the pseudo-second-order model. The equilibrium data revealed that the Langmuir model fitted better. IAO/GO can be applied practically to natural water environments by virtue of its good selectivity for fluoride when co-anions exist, the considerable removal efficiency and low residual iron and aluminum concentration after defluoridation in a wide pH range (3–9). The main adsorption mechanisms of fluoride onto the adsorbent include electrostatic interactions, anion exchange, and inner-sphere complexation.Acknowledgements
This work was supported by the Environmental Protection and Public Welfare Industry Research Special (No. 201109022) and National High-tech Research and Development Projects (No. 2012AA061705).References
- Y.-H. Huang, Y.-J. Shih and C.-C. Chang, J. Hazard. Mater., 2011, 186, 1355–1359 CrossRef CAS PubMed.
- I. Abe, S. Iwasaki, T. Tokimoto, N. Kawasaki, T. Nakamura and S. Tanada, J. Colloid Interface Sci., 2004, 275, 35–39 CrossRef CAS PubMed.
- D. Banks, C. Reimann, O. Røyset, H. Skarphagen and O. M. Sæther, Appl. Geochem., 1995, 10, 1–16 CrossRef CAS.
- L. Lv, J. He, M. Wei, D. G. Evans and X. Duan, J. Hazard. Mater., 2006, 133, 119–128 CrossRef CAS PubMed.
- World Health Organization, Guidelines for Drinking-Water Quality: Incorporating First Addendum Recommendations, 2006, http://www.who.int/water_sanitation_health/dwq/gdwq0506.pdf Search PubMed.
- L. Chai, Y. Wang, N. Zhao, W. Yang and X. You, Water Res., 2013, 47, 4040–4049 CrossRef CAS PubMed.
- J. Ahn, Environ. Geochem. Health, 2012, 34, 43–54 CrossRef CAS PubMed.
- W. Wang, R. Li, J. a. Tan, K. Luo, L. Yang, H. Li and Y. Li, Fluoride, 2002, 35, 122–129 CAS.
- P. I. Ndiaye, P. Moulin, L. Dominguez, J. C. Millet and F. Charbit, Desalination, 2005, 173, 25–32 CrossRef CAS.
- Z. Amor, B. Bariou, N. Mameri, M. Taky, S. Nicolas and A. Elmidaoui, Desalination, 2001, 133, 215–223 CrossRef CAS.
- M. Mohapatra, S. Anand, B. K. Mishra, D. E. Giles and P. Singh, J. Environ. Manage., 2009, 91, 67–77 CrossRef CAS PubMed.
- L. H. Velazquez-Jimenez, R. H. Hurt, J. Matos and J. R. Rangel-Mendez, Environ. Sci. Technol., 2013, 48, 1166–1174 CrossRef PubMed.
- Y. Ku and H.-M. Chiou, Water, Air, Soil Pollut., 2002, 133, 349–361 CrossRef CAS.
- M. G. Sujana, R. S. Thakur and S. B. Rao, J. Colloid Interface Sci., 1998, 206, 94–101 CrossRef CAS PubMed.
- K. Biswas, K. Gupta, A. Goswami and U. C. Ghosh, Desalination, 2010, 255, 44–51 CrossRef CAS.
- X. Wu, Y. Zhang, X. Dou and M. Yang, Chemosphere, 2007, 69, 1758–1764 CrossRef CAS PubMed.
- L. Lv, J. He, M. Wei, D. G. Evans and Z. Zhou, Water Res., 2007, 41, 1534–1542 CrossRef CAS PubMed.
- X.-p. Liao and B. Shi, Environ. Sci. Technol., 2005, 39, 4628–4632 CrossRef CAS PubMed.
- K. Biswas, S. K. Saha and U. C. Ghosh, Ind. Eng. Chem. Res., 2007, 46, 5346–5356 CrossRef CAS.
- M. G. Sujana, G. Soma, N. Vasumathi and S. Anand, J. Fluorine Chem., 2009, 130, 749–754 CrossRef CAS.
- E. Kumar, A. Bhatnagar, M. Ji, W. Jung, S.-H. Lee, S.-J. Kim, G. Lee, H. Song, J.-Y. Choi, J.-S. Yang and B.-H. Jeon, Water Res., 2009, 43, 490–498 CrossRef CAS PubMed.
- X. Zhao, J. Wang, F. Wu, T. Wang, Y. Cai, Y. Shi and G. Jiang, J. Hazard. Mater., 2010, 173, 102–109 CrossRef CAS PubMed.
- R.-r. Shan, L.-g. Yan, K. Yang, S.-j. Yu, Y.-f. Hao, H.-q. Yu and B. Du, Chem. Eng. J., 2014, 252, 38–46 CrossRef CAS.
- M. Bhaumik, T. Y. Leswifi, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 186, 150–159 CrossRef CAS PubMed.
- K. A. Mkhoyan, A. W. Contryman, J. Silcox, D. A. Stewart, G. Eda, C. Mattevi, S. Miller and M. Chhowalla, Nano Lett., 2009, 9, 1058–1063 CrossRef CAS PubMed.
- B. Zawisza, R. Sitko, E. Malicka and E. Talik, Anal. Methods, 2013, 5, 6425–6430 RSC.
- P. Shi, R. Su, F. Wan, M. Zhu, D. Li and S. Xu, Appl. Catal., B, 2012, 123–124, 265–272 CrossRef CAS.
- J. Sun, Q. Liang, Q. Han, X. Zhang and M. Ding, Talanta, 2015, 132, 557–563 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- H. Sun, L. Cao and L. Lu, Nano Res., 2011, 4, 550–562 CrossRef CAS.
- J. Li, S. Zhang, C. Chen, G. Zhao, X. Yang, J. Li and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4991–5000 CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- B. M. Babić, S. K. Milonjić, M. J. Polovina and B. V. Kaludierović, Carbon, 1999, 37, 477–481 CrossRef.
- L. Ai, C. Zhang and Z. Chen, J. Hazard. Mater., 2011, 192, 1515–1524 CrossRef CAS PubMed.
- P. Shi, R. Su, S. Zhu, M. Zhu, D. Li and S. Xu, J. Hazard. Mater., 2012, 229–230, 331–339 CrossRef CAS PubMed.
- Y. Zhang, M. Yang, X.-M. Dou, H. He and D.-S. Wang, Environ. Sci. Technol., 2005, 39, 7246–7253 CrossRef CAS PubMed.
- L. Chen, B.-Y. He, S. He, T.-J. Wang, C.-L. Su and Y. Jin, Powder Technol., 2012, 227, 3–8 CrossRef CAS.
- C. Xu, X. Wang and J. Zhu, J. Phys. Chem. C, 2008, 112, 19841–19845 CAS.
- S. Guo, G. Zhang, Y. Guo and J. C. Yu, Carbon, 2013, 60, 437–444 CrossRef CAS.
- Y. Yao, Z. Yang, H. Sun and S. Wang, Ind. Eng. Chem. Res., 2012, 51, 14958–14965 CrossRef CAS.
- S. A. Kahani and M. Jafari, J. Magn. Magn. Mater., 2009, 321, 1951–1954 CrossRef CAS.
- T. Wen, X. Wu, X. Tan, X. Wang and A. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 3304–3311 CAS.
- Z. Li, S. Deng, G. Yu, J. Huang and V. C. Lim, Chem. Eng. J., 2010, 161, 106–113 CrossRef CAS.
- H. Liu, W. Ning, P. Cheng, J. Zhang, Y. Wang and C. Zhang, J. Anal. Appl. Pyrolysis, 2013, 101, 156–165 CrossRef CAS.
- S. S. Tripathy, J.-L. Bersillon and K. Gopal, Sep. Purif. Technol., 2006, 50, 310–317 CrossRef CAS.
- H. Liu, X. Wang, G. Zhai, J. Zhang, C. Zhang, N. Bao and C. Cheng, Chem. Eng. J., 2012, 209, 155–162 CrossRef CAS.
- M. S. Onyango, Y. Kojima, O. Aoyi, E. C. Bernardo and H. Matsuda, J. Colloid Interface Sci., 2004, 279, 341–350 CrossRef CAS PubMed.
- A. Eskandarpour, M. S. Onyango, A. Ochieng and S. Asai, J. Hazard. Mater., 2008, 152, 571–579 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23676b |
| This journal is © The Royal Society of Chemistry 2016 |