Fabrication and analysis of dye-sensitized solar cells (DSSCs) using porphyrin dyes with catechol anchoring groups†
Maryam Adineha,
Pooya Tahaya,
Mohsen Amerib,
Nasser Safari*a and
Ezeddin Mohajeranic
aDepartment of Chemistry, Shahid Beheshti University, Evin, 1983963113 Tehran, Iran. E-mail: n-safari@sbu.ac.ir
bDepartment of Physics, Bu-Ali Sina University, Hamedan, Iran
cLaser and Plasma Research Institute, Shahid Beheshti University, Tehran, 1983969411, Iran
First published on 8th January 2016
Abstract
Herein we report the preparation and application of 4 different zinc(II) tetrakis(dihydroxyphenyl) porphyrins (ZnTDHPP) as the sensitizing dyes in dye-sensitized solar cells (DSSCs). The experimental results include solution and solid state UV-Vis data, steady state current–voltage characteristics, and our theoretical analysis comprised of density functional theory (DFT) and Langmuir isotherm adsorption formalism. The results show that the Zn tetrakis(2,3-dihydroxyphenyl) porphyrin (Zn2,3TDHPP) and Zn tetrakis(3,4-dihydroxyphenyl) porphyrin (Zn3,4TDHPP) with adjacent hydroxyl groups attaches to a TiO2 surface much more strongly than carboxylate. The catechol anchoring group showed high stability of the dye on the TiO2 surface. The cells prepared from these porphyrins showed no significant desorption of dye from the TiO2 surface after several days. The DSSCs based on Zn2,3TDHPP showed the best photovoltaic performance under AM 1.5 irradiation comparable to the conventional Zn tetrakis(p-carboxyphenyl) porphyrin (ZnTCPP), despite lower dye loading on the TiO2 surface. However, non-cooperative OH bonding to TiO2 for Zn2,4TDHPP and Zn2,5TDHPP shows weak attachment to the TiO2 surface and lower efficiencies. DFT calculations showed that the Zn2,3TDHPP structure is more non-planar than the others, which may suppress dye aggregation. The adopted adsorption modeling is fitted to the experimental data to study the kinetics of dye loading. The study can herald development of a new class of porphyrin sensitizer for DSSC applications.
1 Introduction
With population increase, resource depletion and the rising cost of fossil energy sources, researchers have tried to develop clean and renewable energy such as solar energy. Solar energy is an abundant, renewable and clean energy source that could supply energy for societies. Dye-sensitized solar cells (DSSCs) are the third generation of solar cells, first developed by O'Regan and Gratzel in 1991.1 DSSC generally comprises a photoanode, which is made of a semiconductor, a sensitizer (dye molecules) on a transparent conducting oxide (TCO) substrate and a hole conducting material as electrolyte. Photoexcitation of dye molecules leads to electron injection into the conduction band of the semiconductor metal oxide (generally titanium dioxide), which is in contact with an electrolyte solution, generating the oxidized dye. The process is followed by electron transfer from the electrolyte to the sensitizer and dye regeneration.2–4Inspired by the important role of porphyrins in photosynthesis, many researchers have used porphyrin derivatives as sensitizers in DSSCs.5–14 To date, the highest DSSC efficiency has been reported for a porphyrin dye.12 Porphyrins are macrocyclic compounds with highly π-conjugated systems exhibiting intense absorptions in the visible region. Some functional groups can extend the porphyrin and increase their ability to absorb light and therefore making them good choices for use in solar energy conversion. Catechol is such a functional group, which could extend the porphyrin π-system.15 Catechol porphyrins have potential application in solar cells.16,17 ortho-Dihydroxy phenyl functional groups in catechol porphyrins are directed at the meso positions. ortho Dihydroxy phenyl can easily be oxidized to ortho-quinone by electrochemical methods.18 Porphyrins and catechol are both active in electrochemistry and coupling these two compounds could make new compounds with interesting properties.15–17,19
Anchoring groups play an important role in DSSCs. To have a fast electron injection into the TiO2 semiconductor, the existence of anchoring groups is crucial to attach the dye onto the TiO2 surface. Carboxylate is an anchoring group, which has been widely studied.20–25 The major binding modes for the carboxylate anchoring group are bidentate chelating and bidentate bridging of the carboxylate on the TiO2 surface but an monodentate mode is also possible (Scheme 1).26
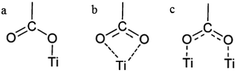 | ||
| Scheme 1 (a) Monodentate, (b) bidentate chelating and (c) bidentate bridging modes of carboxylate binding on a TiO2 surface. | ||
Similar to the carboxylate anchoring group, catechol can also bind to the TiO2 surface in monodentate or bidentate modes. Li et al.19 have shown that catechol can form two full coverage H-bonded structures, containing monodentate only, or mixed monodentate and bidentate molecules on the TiO2 surface (Scheme 2). Monodentate and bidentate structures can simply interconvert via proton exchange between catechol and the TiO2 surface. In the bidentate mode, the formation of both bidentate chelating (Scheme 2b) and bidentate bridging (Scheme 2c) complexes have been reported.27–29
 | ||
| Scheme 2 (a) Monodentate, (b) bidentate chelating and (c) bidentate bridging modes of catechol binding on a TiO2 surface. | ||
In this study, we used zinc(II) tetrakis(dihydroxyphenyl) porphyrin (Zn-TDHPP) as the sensitizer in dye-sensitized solar cells and compared the results with tetrakis(p-carboxyphenyl) zinc(II) porphyrin (ZnTCPP), which has been previously studied.22,30 To the best of our knowledge, this is the first report of a porphyrin dye with a catechol anchoring group thus far. Photosensitizers are characterized using NMR, FT-IR and UV-Vis spectroscopy. To investigate application of these sensitizers in DSSCs, the solution and solid state UV-Vis spectroscopy are used, and the dye loading amount is derived from these experiments. The current–voltage characteristics are also provided for the DSSCs with the synthesized photosensitizers. To obtain further evidence, density functional theory (DFT) calculations were carried out. Dye adsorption modeling was also used to investigate the adsorption kinetics and estimate electron injection efficiencies from dye to the TiO2 surface. General structures of metalloporphyrins used in this study are shown in Scheme 3.
2 Experimental
2.1 Materials and reagents
Tetrakis(dihydroxyphenyl) porphyrins were obtained from tetrakis(dimethoxyphenyl) porphyrins (ZnTDMPP). Tetrakis(dimethoxyphenyl) porphyrins and tetrakis(p-carboxyphenyl) porphyrin were prepared by the condensation of the appropriate dimethoxybenzaldehyde with pyrrole in refluxing propionic acid.27 The tetrakis(dihydroxyphenyl) porphyrins were prepared by boron tribromide demethylation of the corresponding methyl ethers, according to the method presented by Milgrom.31 Solvents were dried over appropriate drying agents. All other chemicals were reagent grade and used without further purification.2.2 Analytical instruments and measurements
Solution absorption spectra were obtained on an Analytik Jena s600 spectrometer using a 1 cm cuvette. Solutions were made at concentrations of 5 × 10−5 M in ethanol. Solid state absorption spectra were obtained on a Shimadzu UV-2100 spectrometer.1H NMR and 13C NMR spectra were obtained in DMSO-d6 using a 300 MHz Bruker instrument. Photocurrent–voltage (I–V) measurements were performed using a Sharif solar (I–V) source measure unit and Prova 8300 sun simulator.
2.3 Synthesis of dyes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluents.
1) as eluents.Tetrakis(3,4-dimethoxyphenyl) porphyrin: yield: 350 mg 4%, 1H NMR (CDCl3, 300 MHz), δ, (ppm): 8.9 (s, 8H), 7.8 (s, 4H), 7.7 (d, 4H), 7.2 (d, 4H), 4.1 (s, 12H); 3.9 (s, 12H), −2.70 (s, 2H). 13C NMR (CDCl3, 100 MHz), δ, (ppm): 148.8, 147.0, 134.7, 131.1, 130.0, 127.34, 126.7, 119.8, 118.2, 110.2, 109.4, 108.7, 56.0, 55.9; UV-Vis, (CHCl3 λmax): 421 (Soret); 518; 556; 592; 649.
Tetrakis(2,3-dimethoxyphenyl) porphyrin: yield: 680 mg 8%, 1H NMR (300 MHz, CDCl3, 25 °C): δ, (ppm), 8.7 (s, 8H), 7.2 (m, 4H), 7.1 (d, 4H), 7.0 (m, 4H), 3.9 (s, 12H), 1.8 (s, 12H), −2.8 (s, 2H).
Tetrakis(2,4-dimethoxyphenyl) porphyrin: yield: 682 mg 8%, 1H NMR (CDCl3, 300 MHz), δ, (ppm), 8.7 (s, 8H), 7.7 (s, 4H), 7.0 (s, 8H), 4.1 (s, 12H), 1.8 (s, 12H), −2.7 (s, 2H).
Tetrakis(2,5-dimethoxyphenyl) porphyrin: yield: 500 mg 6%, 1H NMR (CDCl3, 300 MHz), δ, (ppm), 9 (s, 8H), 7.4 (d, 4H), 7.1 (s, 8H), −2.8 (s, 2H), 3.9 (s, 12H), 1.9 (s, 12H).
Yield: 10%, 1H NMR (300 MHz, DMSO-d6, 25 °C) δ (ppm): 8.7 (s, 8H), 8.3 (d, 8H), 8.1 (d, 8H), −2.9 (s, 2H).
ZnTCPP: yield: 94%, 1H NMR (300 MHz, DMSO-d6, 25 °C) δ (ppm): 8.9 (s, 8H), 8.4 (d, 8H), 8.3 (d, 8H). UV-Vis (EtOH, λmax, nm): 425 (Soret), 558, 601.
2,3TDHPP: yield: 80%, 1H NMR (300 MHz, DMSO-d6, 25 °C): δ, (ppm) 9.7 (s, 8H), 8.7 (s, 8H), 7.2 (m, 4H), 7.1 (d, 4H), 7.0 (m, 4H), −2.8 (s, 2H). 13C NMR (75 MHz, DMSO-d6, 25 °C): δ, (ppm), 146.5, 145.5, 129.1, 128.6, 127.8, 126.8, 118.1, 116.8, 116.1. IR (KBr): ν, cm−1, 3423, 2923, 2853, 1615, 1586, 1466, 1346, 1204, 1145, 927, 844, 804, 768, 730. UV-Vis (EtOH, λmax, nm): 420 (Soret), 516, 550, 591, 647.
2,4TDHPP: yield: 78%, 1H NMR (300 MHz, DMSO-d6, 25 °C): δ, (ppm) 9.1 (s, 8H), 8.8 (s, 8H), 7.7 (s, 4H), 7.0 (s, 8H), −2.7 (s, 2H). 13C NMR (75 MHz, DMSO-d6, 25 °C): δ, (ppm), 146.5, 145.5, 129.1, 128.6, 127.8, 126.8, 118.1, 116.8, 116.1.
Zn2,3TDHPP: yield: 95%, 1H NMR (300 MHz, DMSO-d6, 25 °C): δ, (ppm) 9.7 (s, 8H), 8.8 (s, 8H), 7.4 (m, 4H), 7.2 (d, 4H), 7.0 (m, 4H). 13C NMR (75 MHz, DMSO-d6, 25 °C): δ, (ppm), 146.6, 145.5, 129.2, 128.8, 128.0, 126.8, 118.3, 116.9, 115.9. IR (KBr): ν, cm−1, 3426, 2926, 2851, 1620, 1586, 1466, 1346, 1210, 1145, 927, 844, 809, 768, 735, 547, 469. UV-Vis (EtOH, λmax, nm): 425 (Soret), 556, 592. Elemental analysis calc. for C44H28N4O8 (%) Zn: C, 65.56; H, 3.50; N, 6.95; found (%) C, 65.58; H, 3.45; N, 6.98.
Zn3,4TDHPP: yield: 95%, 1H NMR (300 MHz, DMSO-d6, 25 °C): δ, (ppm) 9.4 (s, 8H), 8.8 (s, 8H), 7.7 (s, 4H), 7.4 (s, 4H), 7.1 (s, 4H). 13C NMR (75 MHz, DMSO-d6, 25 °C): δ, (ppm) 145.0, 144.1, 132.8, 131.6, 126.7, 122.9, 120.4, 115.9, 114.7. UV-Vis (EtOH, λmax, nm): 428 (Soret), 559, 602.
Zn2,4TDHPP: yield: 96%, 1H NMR (300 MHz, DMSO-d6, 25 °C): δ, (ppm) 9.4 (s, 8H), 8.8 (s, 8H), 7.7 (s, 4H), 6.9 (s, 8H). UV-Vis (EtOH, λmax, nm): 425 (Soret), 556, 597.
Zn2,5TDHPP: yield: 95%, 1H NMR (300 MHz, DMSO-d6, 25 °C): δ, (ppm) 9.7 (s, 8H), 9 (s, 8H), 7.4 (d, 4H), 7.1 (s, 8H). UV-Vis (EtOH, λmax, nm): 424 (Soret), 556, 591.
2.4 Fabrication of dye-sensitized solar cells and photovoltaic measurements
Paste B, which was used in the light-scattering layers, was prepared with the same method using 350 nm TiO2 nanoparticles.
The TiO2 films were once again treated with 40 mM TiCl4 solution, as previously described, then rinsed with water and methanol and heated at 450 °C for 15 min and 500 °C for 15 min. After cooling to 80 °C, the TiO2 electrodes were immersed into 0.1 mM dye solution with 35 mM 4-tert-butylpyridine as additive in a mixture of ethanol and THF (volume ratio, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and maintained at room temperature for 2 h to take up the dye.
1) and maintained at room temperature for 2 h to take up the dye.
Clamps assembled the dye-adsorbed TiO2 electrode and counter electrode into a sandwich-type cell. Finally, a drop of electrolyte solution (0.10 M lithium iodide, 0.60 M butylmethylimidazolium iodide, 0.03 M I2, and 0.05 M 4-tert-butylpyridine in acetonitrile/valeronitrile 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) was introduced into the clamped electrodes.
25) was introduced into the clamped electrodes.
2.5 Desorption measurement
The loading of each dye was determined by dye desorption in a basic solution. The dye-loaded anodes were soaked in a 10% solution of tetrabutylammonium hydroxide in water/EtOH. The solution absorbances were measured with time until the dyes completely desorbed from electrode.3 Results and discussion
We have studied four types of porphyrin dyes, which are Zn2,3TDHPP, Zn2,4TDHPP, Zn2,5TDHPP, and Zn3,4TDHPP, and compared them with previously studied dye ZnTCPP (Scheme 3). Each of these dyes was chosen because they have two hydroxyl groups on each porphyrin meso-phenyl as anchoring groups. In the case of Zn2,3TDHPP and Zn3,4TDHPP these two hydroxyl groups were adjacent and cooperatively bond to the TiO2 surface, but for Zn2,4TDHPP and Zn2,5TDHPP they were non-adjacent.3.1 Photophysical properties of the dyes in solution and on TiO2 film
Zinc is a closed shell metal and has very little effect on the porphyrin π to π* energy gap in porphyrin electronic spectra. The Soret bands for ZnTDHPPs were 5–8 nm red-shifted relative to zinc tetraphenylporphyrin, which indicates the conjugation of the lone pair electrons of the eight oxygen atoms that are directly attached to the phenyl rings, with π electrons of the macrocycle rings.18,34 Extinction coefficients for ZnTDHPPs were lower than ZnTPP due to both electronic and steric effects.35,36 ZnTDHPPs with adjacent hydroxyl groups have relatively higher extinction coefficients than the porphyrins with non-adjacent hydroxyl groups. The lower extinction coefficient in such porphyrins, i.e. Zn2,5TDHPP, could be due to the resonance forms shown in Scheme 4.37 The π system extension, results in a red-shift in the porphyrin UV-Vis spectra and the electronegativity of oxygen attracts electron density from the porphyrin ring and lowers the extinction coefficient.36
ZnTCPP and porphyrins with non-adjacent hydroxyl groups desorbed from TiO2 surface very quickly; in comparison, Zn2,3TDHPP and Zn3,4TDHPP desorbed more slowly from the TiO2 surface. Zn2,4TDHPP and Zn2,5TDHPP almost completely desorbed from the TiO2 surface after 30 minutes and ZnTCPP after two hours yet this process took six hours for Zn2,3TDHPP and Zn3,4TDHPP. There was no significant desorption in acidic and neutral media after days. Therefore, Zn2,3TDHPP and Zn3,4TDHPP showed almost three times more stability on the TiO2 surface. This significant increase in stability of the catechol anchoring group can arise from cooperative bonding of dihydroxy phenyl via a stable five-membered ring (form b in Scheme 2).
Table 1 shows data concerning spectral properties and dye loading amount of all the synthesized porphyrin derivatives on the TiO2 surface calculated from desorption and calibration data.
| Dye | λmax (nm) ethanol | ε × 10−5 (M−1 cm−1) | λmax (nm) on TiO2 surface | Dye loading (nmol cm−2) | Injection efficiency% (φinj) | Adsorption coefficient (ka) nmol cm−1 | Desorption coefficient (kd) nmol cm−1 |
|---|---|---|---|---|---|---|---|
| ZnTCPP | 425 | 3.466 | 425 | 33.5 | 2.63 | 100 × 10−3.0 | 2.85 × 10−3.9 |
| Zn2,3TDHPP | 425 | 4.538 | 430 | 25.3 | 2.6 | 100 × 10−3.1 | 3.5 × 10−3.9 |
| Zn3,4TDHPP | 428 | 3.098 | 435 | 36.7 | 2.5 | 100 × 10−3.01 | 2.85 × 10−3.9 |
| Zn2,5TDHPP | 424 | 2.84 | 429 | 15.1 | 0.9 | 57 × 10−3.01 | 3.8 × 10−3.75 |
| Zn2,4TDHPP | 425 | 2.24 | 432 | 10.4 | 0.5 | 55 × 10−3.01 | 2.5 × 10−3.75 |
For non-adjacent hydroxyl group dyes, the loading amount and also desorption time were very low, which indicates that in such porphyrins, there was no effective attachment of dye on the TiO2 surface. Although all the electrodes were soaked in dye solutions of the same concentration for the same period of time, the amount of adsorbed dye for Zn2,3TDHPP was lower than that for Zn3,4TDHPP. This observation suggests that the flat orientation of adsorbed porphyrin with respect to the TiO2 surface of Zn2,3TDHPP (25.3 nmol cm−2) results in less dye uptake compared to Zn3,4TDHPP (36.7 nmol cm−2) and ZnTCPP (33.5 nmol cm−2)20,43 (Fig. 4).
Herein, we implement a model, developed in our previous study,44 for investigation of time evolution of dye loading to extract the dye adsorption. The adsorption from dye solution by solid nanoparticles is studied assuming a kinetic form of the time-dependent Langmuir formalism framework. In the present microscopic model, it is assumed that the bulk density of dye molecules are adsorbed only by the nanoparticles network by a constant adsorption and desorption rates, also the interconnection of nanoparticles is influenced by particle necking, which leads to partial aggregation of nanoparticles.
Using ka and kd as the rate constants for adsorption and desorption, respectively, we can write the net rate of adsorption for solution phase species i as the difference between the rate of adsorption and the rate of desorption as follows:
| Ri,ads,net = kaiCisol(Ci-site,total − Ci-sites,occupied by i) − kd,sCi-sites,occupied by i | (1) |
Solution phase
 | (2) |
Solid phase
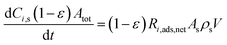 | (3) |
| φinj = φ(1 − 10−Ca) | (4) |
The highest rate of injection efficiency belongs to ZnTCPP. The estimated injection quantum yield together with 100% collection efficiency in DSSCs strongly suggest that the limiting performance factor for DSSCs can be the inefficient injection efficiency of the cells (Table 1).
3.2 Photovoltaic properties
To consider the potential of ZnTDHPP as photosensitizers for DSSCs, their DSSC performances were tested. Fig. 5 shows J–V curves for the studied dyes. Detailed photovoltaic parameters, such as open circuit voltage (VOC), short circuit current (JSC), fill factor (ff) and power conversion efficiency (η) for different dyes under AM 1.5 solar illumination, have been listed in Table 2. Power conversion efficiency is derived from the equation: η = JSC × VOC × ff, where JSC is the short circuit current, VOC is the open circuit potential, and ff is the fill factor. | ||
| Fig. 5 Current–voltage curves for DSSCs based on Zn2,3TDHPP, Zn3,4TDHPP, Zn2,4TDHPP and Zn2,5TDHPP under illumination of AM 1.5 simulated sunlight. | ||
| Dye | VOC (mV) | JSC (mA cm−2) | Fill factor (%) | Efficiency (%) |
|---|---|---|---|---|
| ZnTCPP | 449 | 2.7 | 67 | 0.8 |
| Zn2,3TDHPP | 449 | 2.1 | 62 | 0.6 |
| Zn3,4TDHPP | 410 | 1.11 | 51 | 0.23 |
| Zn2,4TDHPP | 312 | 0.28 | 34 | 0.03 |
| Zn2,5TDHPP | 390 | 0.12 | 38 | 0.02 |
The VOC values of all the dye cells ranked: ZnTCPP = Zn2,3TDHPP > Zn3,4TDHPP > Zn2,5TDHPP > Zn2,4TDHPP. Despite the lower dye loading amount for Zn2,3TDHPP compared to ZnTCPP and Zn3,4TDHPP, the VOC value for this porphyrin is similar to ZnTCPP. This could be because of the flat orientation of Zn2,3TDHPP on the TiO2 surface, meaning it could cover more of the surface than the other dyes and prevent direct contact of electrolyte with TiO2 as well as decrease the recombination.
Similar to the power conversion efficiencies, the JSC values of all the dye cells ranked: ZnTCPP > Zn2,3TDHPP > Zn3,4TDHPP > Zn2,4TDHPP > Zn2,5TDHPP. The order is the same as extinction coefficient for ZnTDHPPs, indicating that short circuit current increases with increase in light absorption ability. However, in the case of Zn2,4TDHPP and Zn2,5TDHPP photovoltaic results were poor because of weak binding on the TiO2 surface and in some extent due to lower extinction coefficients of the corresponding dye.
3.3 Density functional theory (DFT) calculations
To obtain further evidence for the abovementioned results, DFT calculations of two porphyrins with adjacent hydroxyl group and ZnTCPP dyes were carried out, using the B3LYP/3-21G level. The calculated structures do not show negative frequencies, inferring that the optimized geometries are in the global energy minima. Fig. 6 illustrates the electron density distributions of dyes in their respective HOMO and LUMO levels. The energy levels of frontier orbitals and the HOMO–LUMO energy gaps for these three dyes are listed in Table 3.| Dye | HOMO (eV) | LUMO (eV) | Eg (eV) |
|---|---|---|---|
| ZnTCPP | −5.34 | −2.40 | 2.94 |
| Zn2,3TDHPP | −4.95 | −1.91 | 3.03 |
| Zn3,4TDHPP | −4.85 | −1.95 | 2.90 |
Electron distributions of all three dyes, in the frontier orbitals, are mostly over the porphyrin ring, which is likely due to the fact that the phenyl rings are perpendicular to the porphyrin ring and do not contribute to electron distributions. This could block electron transfer to TiO2 and reduce photovoltaic performance for studied dyes to those that have anchoring group participation in electron conjugation. It is well known that the electron density distribution of the LUMO around an anchoring group affects the electronic coupling between the excited adsorbed dye and 3d orbital of TiO2.45 Consequently, we can expect the low φinj values of dyes (Table 1).
For ZnTDHPPs, variations in energies for different positions of the hydroxyl groups for the HOMO level (−4.95 eV for Zn2,3TDHPP and −4.85 eV for Zn3,4TDHPP) are larger than those for the LUMO level (−1.91 eV for Zn2,3TDHPP and −1.95 eV for Zn3,4TDHPP) indicating that variation in the hydroxyl group position mainly affect the occupied orbitals of ZnTDHPP dyes (Table 3). In addition, the dihedral angle between the phenyl unit and the porphyrin macrocycle for Zn2,3TDHPP is 84.1° but the corresponding angles for Zn3,4TDHPP and ZnTCPP are 69.1° and 69.5°, respectively, indicating that Zn2,3TDHPP is more non-planar than the others.
It is well known that for non-planar and distorted dyes, dye aggregation and charge recombination can be efficiently suppressed, which is favorable for enhancing cell efficiency.42 Similarly, the lower VOC for Zn3,4TDHPP could be a consequence of the lower dihedral angle between the phenyl unit and the porphyrin macrocycle and the more planar structure that favors aggregation. Therefore, Zn2,3TDHPP compared to Zn3,4TDHPP could be considered a better dye due to its different mode of attachment (Fig. 4) and its relatively non-planar structure.8,42,46
To have fast electron transfer, the HOMO must be sufficiently more positive than the redox potential of I−/I3− to accept electrons effectively; the difference between these two levels is given by ΔE.47 HOMO energy levels are −5.34, −4,95 and −4.85 for ZnTCPP, Zn2,3TDHPP and Zn3,4TDHPP, respectively; considering that I−/I3− electrolyte potential is −4.85 eV,48 the differences between electrolyte and HOMO energy level (ΔE) are 0.5, 0.1 and 0 for ZnTCPP, Zn2,3TDHPP and Zn3,4TDHPP, respectively. For Zn3,4TDHPP, there is no driving force for electron injection from electrolyte to dye oxidized state, which could be the reason for poor photocurrent in this dye (Fig. 7).47
 | ||
| Fig. 7 Illustration of energy levels and electron injection efficiencies of ZnTCPP, Zn2,3TDHPP and Zn3,4TDHPP. | ||
4 Conclusion
ZnTDHPP dyes were successfully synthesized and used as sensitizers in DSSCs and compared with ZnTCPP as the reference dye. This is the first report of a porphyrin dye with a catechol anchoring group to date. The JSC values of all the dye cells, as well as the power conversion efficiencies, were ranked: ZnTCPP > Zn2,3TDHPP > Zn3,4TDHPP > Zn2,4TDHPP > Zn2,5TDHPP; the order was the same as extinction coefficients for ZnTDHPPs. Despite lower dye loading on the TiO2 surface for Zn2,3TDHPP, it has comparable photovoltaic performance (0.6 vs. 0.9) and more stability than ZnTCPP. The stability of the dye on TiO2 for Zn2,3TDHPP and Zn3,4TDHPP might arise from bidentate coordination on the TiO2 surface via chelation and formation of five-membered rings. The higher efficiency observed for Zn2,3TDHPP relative to Zn3,4TDHPP may be ascribed to the mode of coordination to the TiO2 surface which results in more efficient electron transfer, suppressed dye aggregation and high extinction coefficient for the former compound. Injection efficiencies of the cells were also calculated; the results indicate that the limiting performance factor for DSSCs can be the inefficient injection efficiency of the cells. Our future strategy is to take advantage of the high stability of the catechol anchoring group to extend the π system contribution and also to synthesise asymmetric porphyrins to make dipole moments by modification of the porphyrin core to enhance the efficiencies.Acknowledgements
This study was supported by the Iranian National Science Foundation (INSF) and Shahid Beheshti University Research Affaires.References
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- M. K. Nazeeruddin, E. Baranoff and M. Grätzel, Solar Energy, 2011, 85, 1172–1178 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- N. Xiang, X. Huang, X. Feng, Y. Liu, B. Zhao, L. Deng, P. Shen, J. Fei and S. Tan, Dyes Pigm., 2011, 88, 75–83 CrossRef CAS.
- A. Yella, H. W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. G. Diau, C. Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- S. Eu, S. Hayashi, T. Umeyama, Y. Matano, Y. Araki and H. Imahori, J. Phys. Chem. C, 2008, 112, 4396–4405 CAS.
- H.-P. Wu, Z.-W. Ou, T.-Y. Pan, C.-M. Lan, W.-K. Huang, H.-W. Lee, N. M. Reddy, C.-T. Chen, W.-S. Chao, C.-Y. Yeh and E. W.-G. Diau, Energy Environ. Sci., 2012, 5, 9843–9848 CAS.
- D. Arteaga, R. Cotta, A. Ortiz, B. Insuasty, N. Martin and L. Echegoyen, Dyes Pigm., 2015, 112, 127–137 CrossRef CAS.
- T. Hasobe, S. Hattori, P. V. Kamat, Y. Urano, N. Umezawa, T. Nagano and S. Fukuzumi, Chem. Phys., 2005, 319, 243–252 CrossRef CAS.
- A. Yella, C.-L. Mai, S. M. Zakeeruddin, S.-N. Chang, C.-H. Hsieh, C.-Y. Yeh and M. Grätzel, Angew. Chem., 2014, 126, 3017–3021 CrossRef.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- D. Daphnomili, G. D. Sharma, S. Biswas, K. R. Justin Thomas and A. G. Coutsolelos, J. Photochem. Photobiol., A, 2013, 253, 88–96 CrossRef CAS.
- H. Deng, Z. Lu, Y. Shen, H. Mao and H. Xu, Chem. Phys., 1998, 231, 95–103 CrossRef CAS.
- R. Mosurkal, J.-A. He, K. Yang, L. A. Samuelson and J. Kumar, J. Photochem. Photobiol., A, 2004, 168, 191–196 CrossRef CAS.
- S. Osati, S. S. H. Davarani, N. Safari and M. H. Banitaba, Electrochim. Acta, 2011, 56, 9426–9432 CrossRef CAS.
- S. Osati, N. Safari, M. K. Bojdi and S. S. H. Davarani, J. Electroanal. Chem., 2011, 655, 120–127 CrossRef CAS.
- S. Shahrokhian and A. Hamzehloei, Electrochem. Commun., 2003, 5, 706–710 CrossRef CAS.
- S.-C. Li, J.-G. Wang, P. Jacobson, X. Q. Gong, A. Selloni and U. Diebold, J. Am. Chem. Soc., 2009, 131, 980–984 CrossRef CAS PubMed.
- W. M. Campbell, A. K. Burrell, D. L. Officer and K. W. Jolley, Coord. Chem. Rev., 2004, 248, 1363–1379 CrossRef CAS.
- J. Rochford, D. Chu, A. Hagfeldt and E. Galoppini, J. Am. Chem. Soc., 2007, 129, 4655–4665 CrossRef CAS PubMed.
- S. Cherian and C. C. Wamser, J. Phys. Chem. B, 2000, 104, 3624–3629 CrossRef CAS.
- A. Kay and M. Graetzel, J. Phys. Chem., 1993, 97, 6272–6277 CrossRef CAS.
- K. Kalyanasundaram, N. Vlachopoulos, V. Krishnan, A. Monnier and M. Graetzel, J. Phys. Chem., 1987, 91, 2342–2347 CrossRef CAS.
- H. Imahori, S. Hayashi, H. Hayashi, A. Oguro, S. Eu, T. Umeyama and Y. Matano, J. Phys. Chem. C, 2009, 113, 18406–18413 CAS.
- M. K. Nazeeruddin, R. Humphry-Baker, P. Liska and M. Grätzel, J. Phys. Chem. B, 2003, 107, 8981–8987 CrossRef CAS.
- I. A. Janković, Z. V. Šaponjić, M. I. Čomor and J. M. Nedeljković, J. Phys. Chem. C, 2009, 113, 12645–12652 Search PubMed.
- P. Z. Araujo, P. J. Morando and M. A. Blesa, Langmuir, 2005, 21, 3470–3474 CrossRef CAS PubMed.
- P. Z. Araujo, C. B. Mendive, L. A. G. Rodenas, P. J. Morando, A. E. Regazzoni, M. A. Blesa and D. Bahnemann, Colloids Surf., A, 2005, 265, 73–80 CrossRef CAS.
- J. Jasieniak, M. Johnston and E. R. Waclawik, J. Phys. Chem. B, 2004, 108, 12962–12971 CrossRef CAS.
- L. R. Milgrom, J. Chem. Soc., Perkin Trans. 2, 1983, 2535–2539, 10.1039/p19830002535.
- S. Ito, T. N. Murakami, P. Comte, P. Liska, C. Grätzel, M. K. Nazeeruddin and M. Grätzel, Thin Solid Films, 2008, 516, 4613–4619 CrossRef CAS.
- M. Späth, P. M. Sommeling, J. A. M. van Roosmalen, H. J. P. Smit, N. P. G. van der Burg, D. R. Mahieu, N. J. Bakker and J. M. Kroon, Prog. Photovoltaics, 2003, 11, 207–220 Search PubMed.
- A. Stone and E. B. Fleischer, J. Am. Chem. Soc., 1968, 90, 2735–2748 CrossRef CAS.
- M. Rojkiewicz, P. Kuś, P. Kozub and M. Kempa, Dyes Pigm., 2013, 99, 627–635 CrossRef CAS.
- J. P. Lewtak, D. Gryko, D. Bao, E. Sebai, O. Vakuliuk, M. Scigaj and D. T. Gryko, Org. Biomol. Chem., 2011, 9, 8178–8181 CAS.
- D. A. James, D. P. Arnold and P. G. Parsons, Photochem. Photobiol., 1994, 59, 441–447 CrossRef CAS PubMed.
- C.-L. Wang, Y.-C. Chang, C.-M. Lan, C.-F. Lo, E. Wei-Guang Diau and C.-Y. Lin, Energy Environ. Sci., 2011, 4, 1788–1795 CAS.
- Y. Ooyama, T. Yamada, T. Fujita, Y. Harima and J. Ohshita, J. Mater. Chem. A, 2014, 2, 8500–8511 CAS.
- P. Péchy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613–1624 CrossRef.
- F. Nüesch and M. Grätzel, Chem. Phys., 1995, 193, 1–17 CrossRef.
- Y. Wang, X. Li, B. Liu, W. Wu, W. Zhu and Y. Xie, RSC Adv., 2013, 3, 14780–14790 RSC.
- A. S. Hart, C. B. Kc, H. B. Gobeze, L. R. Sequeira and F. D'Souza, ACS Appl. Mater. Interfaces, 2013, 5, 5314–5323 CAS.
- M. Ameri and E. Mohajerani, RSC Adv., 2015, 5, 92690–92706 RSC.
- H. Imahori, Y. Matsubara, H. Iijima, T. Umeyama, Y. Matano, S. Ito, M. Niemi, N. V. Tkachenko and H. Lemmetyinen, J. Phys. Chem. C, 2010, 114, 10656–10665 CAS.
- J. H. Delcamp, Y. Shi, J.-H. Yum, T. Sajoto, E. Dell'Orto, S. Barlow, M. K. Nazeeruddin, S. R. Marder and M. Grätzel, Chem.–Eur. J., 2013, 19, 1819–1827 CrossRef CAS PubMed.
- Handbook of Photovoltaic Science and Engineering, 2nd edn, 2011 Search PubMed.
- G. Boschloo and A. Hagfeldt, Acc. Chem. Res., 2009, 42, 1819–1826 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23584g |
| This journal is © The Royal Society of Chemistry 2016 |







