Selective prototropism of lumichrome in cationic micelles and reverse micelles: a photophysical perspective†
Abstract
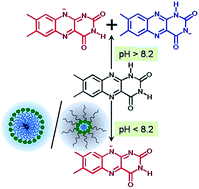
This study reveals the intriguing photophysics of lumichrome (LC) in two types of organized assemblies, namely, normal micelles and reverse micelles, formed by the cationic surfactant, benzyldimethylhexadecylammonium chloride (BHDC). Although LC exists in alloxazine form at around neutral pH in aqueous solutions, in BHDC micelles and reverse micelles under similar pH conditions, LC undergoes selective deprotonation at its N-1 position to generate LC anion in the tautomerically stabilized isoalloxazine form (N10 anion). Considering that deprotonation at both N-1 and N-3 positions of LC are equally feasible (similar pKa) in water at high pH, the preferential formation of N10 anion rather than N3 anion is a unique phenomenon realized in BHDC organized assemblies. This selective prototropism occurs due to combined electrostatic effects of the cationic head groups of BHDC and steric constraints that determine the orientation of LC at the micellar interface. The study also reveals that despite being formed by the same surfactant, BHDC micelles and reverse micelles possess different interfacial structures and hydration characteristics that induce distinct photophysical changes of LC in the two assemblies.

 Please wait while we load your content...
Please wait while we load your content...