Carbon nano onions cross the blood brain barrier†
Abstract
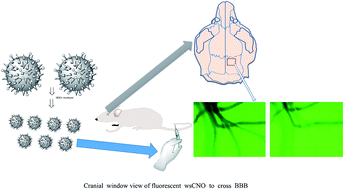
We show the crossing of small sized water soluble fluorescent carbon nano onion (wsCNO) through the blood brain barrier (BBB) in the Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) murine model as well as in glioblastoma multiforme (GBM) induced mice. It is readily excreted from the body after a few days suggesting its possible use as cargo in drug delivery.

 Please wait while we load your content...
Please wait while we load your content...