Synthesis of {110}-faceted rutile TiO2 nanocrystals from tetratitanate nanoribbons for improving dye-sensitized solar cell performance
Abstract
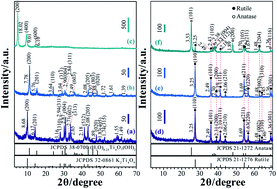
{110}-faceted rutile TiO2 nanocrystals with nanorods and nanoflower morphologies were synthesized through simple hydrothermal treatment of a tetratitanate nanoribbons precursor. The nanostructures and the formation reaction mechanism of the rutile TiO2 nanocrystals from the layered tetratitanate nanoribbons were investigated using X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), transmission electron microscope (TEM) and selected-area electron diffraction (SAED). The transformation from the layered phase into the rutile TiO2 may experience several stages during the hydrothermal reaction including dissolution of nanoribbons, recrystallization of [Ti(OH)6]2− octahedral fragments, and part dissolution and aggregation of the rutile TiO2 nanorods. Furthermore, the dye-sensitized solar cell (DSSC) performance of the synthesized rutile nanocrystals was also characterized, which showed a superior photovoltaic performance, compared to the benchmark P25 TiO2.

 Please wait while we load your content...
Please wait while we load your content...