The anticancer agent ellipticine binds to glycosaminoglycans at mildly acidic pH characteristic of the extracellular matrix of tumor tissues†
Ferenc Zsila*
Biomolecular Self-Assembly Group, Institute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, POB 289, H-1519, Budapest, Hungary. E-mail: zsila.ferenc@ttk.mta.hu
First published on 22nd December 2015
Abstract
In the first instance, this communication demonstrates the glycosaminoglycan (GAG) binding of the anticancer plant alkaloid ellipticine. Its association to heparin and heparan sulfate induces UV hypochromism and a biphasic circular dichroism (CD) signature originating from alkaloid–alkaloid chiral intermolecular exciton coupling. As the pH dependence of the CD signals showed, only the protonated form of ellipticine is capable to bind to the GAG chains. The binding is most prominent in the pH range of 6–7 which corresponds to the mildly acidic extracellular milieu of solid tumors and inflamed tissues. This implies that besides DNA intercalation, ellipticine may exert additional biological effects by targeting selectively the dynamic GAG–protein interaction network of the acidic tumor microenvironment that is crucial in the maintenance of the malignant phenotype.
The 6H-pyrido[4,3-b]carbazole alkaloid ellipticine (Scheme 1) was first isolated from the leaves of the small evergreen tree Ochrosia elliptica (Apocynaceae) indigenous to tropical Asia, Pacific Islands and Australia. Due to its size, planarity and crescent shape, ellipticine is particularly suited for DNA intercalation and thus exhibits anticancer activity.1,2 A great deal of research into its DNA binding properties with relation to the DNA conformation, affinity and unwinding angle has been carried out.3–6 The basic, pyridine-like nitrogen of ellipticine has a pKa value of 7.4.7,8 Accordingly, at physiological pH ellipticine exists in two equilibrate forms, a water soluble cationic and an insoluble neutral species (Scheme 1). In healthy conditions, pH of the extravascular fluid is between 7.2–7.6. Due to the accumulation of acidic metabolites (e.g., lactic acid) produced by the high-rate anaerobic glycolysis of cancer cells, the extracellular pH of malignant tissues is significantly lower ranging from 5.6 to 7.2 with an average value of 6.8.9–11 Moreover, cancer cells in relation to normal ones have a higher intracellular pH of >7.4. Evidence is accumulating that such dysregulated extra- and intracellular pH profiles are associated with cancer malignancy by stimulating proliferation, migration and metastatic spread of tumor cells.12–14 Of note, actively metabolizing immune cells may also trigger local acidification in ischemic tissues and at inflammatory sites.15 Since molecules penetrate across biological membranes most efficiently in neutral form, the acidic extracellular microenvironment may hinder cell uptake of weakly basic compounds having a pKa of 7 and above.16,17 On the other hand, positively charged small molecules can form complexes with the polyanionic, heterogeneously sulfated glycosaminoglycans (GAG) which are ubiquitous both in the extracellular matrix and on cell surfaces (Scheme 2).18–20 Except for heparin found primarily inside cytosolic granules of mast cells, GAG chains including heparan-, chondroitin-, dermatan-, and keratan sulfate are covalently attached to a core protein forming proteoglycans.21,22 In recent years, a plethora of experimental data have been collected emphasizing the prominent role of extracellular proteoglycans in malignant transformation and tumor progression.23–26
Taking these data into consideration, it is reasonable to consider the GAG binding potential of ellipticine. Though it lacks chirality, optical activity can be conferred by its accommodation to the asymmetric GAG chains. By measuring induced signals, circular dichroism (CD) spectroscopy is a well suited technique for detection and characterization of small molecule–GAG complexes.27–29 Above 210 nm, the optically transparent GAG chains have no CD contribution and thus do not perturb the induced CD (ICD) bands.
To simulate the acidic intratumor microenvironment, fixed concentration of heparin and heparan sulfate (Scheme 2) in pH 6.3 Tris–HCl buffer solution (100 mM NaCl) was titrated by increasing amounts of ellipticine. Considering the pKa of 7.4,7,8 the Henderson–Hasselbalch relationship predicts that at this pH value the relative contribution of the protonated form of ellipticine is 93%. Consequently, the UV spectrum is dominated by the cationic species7 which displays two featureless absorption peaks around 240 and 300 nm, respectively (Fig. 1). As linear dichroism measurements showed, the corresponding π–π* transitions are oriented along the long axis of the molecule.30,31 Upon addition of ellipticine to the GAG samples, four alternating, positive/negative–positive/negative CD signals were observed allied to the respective UV bands (Fig. 1). The longer-wavelength band pair consists of an intense positive and a much weaker negative branch while signals of the second couplet at 255 and 231 nm are equally intense. Overall shape, position and intensity ratio of the ICD bands remained invariant over the titration procedure. Such a CD feature reflects chiral intermolecular exciton coupling between ellipticine molecules bound adjacently to the helical GAG chains28,29 and not the accommodation of single molecules to isolated sites in an asymmetric environment. The alkaloid units interact with the asymmetrically disposed sulfate/carboxylate groups via ionic and H-bonds in a manner that results in an array having a net chirality. Employing the exciton coupling theory,32,33 the positive–negative ICD signatures (Fig. 1) refers to the right-handed (P-helical) relative orientation of the long axes of proximal ellipticine molecules. The magnitude and broadening of the positive portion of the longer-wavelength exciton doublet suggests the contribution of an additional, optically active π–π* transition above 300 nm being unresolved both in the CD and the absorption spectrum. The so called g number or anisotropy factor is the dimensionless ratio of the circular dichroic to isotropic absorbance: g = Δε/ε.34 It can be used as a diagnostic tool to locate masked CD transitions. The pattern of the g function of ellipticine–heparan sulfate complexes above 290 nm clearly deviates from that of the corresponding CD curve displaying a second maximum at 319 nm (Fig. 1). Consistently, an additional positive CD feature centered around this wavelength can be accounted for by the asymmetric shape of the longest-wavelength ICD peak. It is to be noted that upon intercalation of ellipticine into DNA base pairs, this π–π* transition also acquires CD activity exhibiting a positive band around 320 nm.35
Comparison of the molar absorption coefficients (εmax) of ellipticine calculated at same concentrations in the presence and absence of heparin indicates hypochromism (Fig. S1, ESI†), that is the absorption spectroscopic manifestation of the interaction between transition dipole moments of proximal chromophores.36,37 In analogy with the strong UV hypochromism of the stacked base pairs in the DNA double helix,38,39 the εmax value of ellipticine decreases due to the stacking-like superposition of the molecules bound to the GAG chains. The progressive decline of the absorption intensity obtained upon raising the alkaloid concentration (Fig. S1, ESI†) can be attributed to the self-association of the increasing fraction of free ellipticine molecules.40
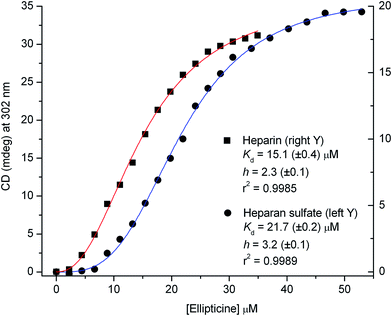
The CD data recorded around 302 nm during the titrations were subjected to non-linear regression analysis to estimate the binding parameters (see ESI†). The sigmoidal shape and the Hill coefficients of the plots suggest the cooperative binding of ellipticine (2 and 3 molecules per a disaccharide unit) that occurs with a somewhat higher affinity to heparin than heparan sulfate (Fig. 2).
In order to assess the influence of pH on ellipticine–GAG interaction, a CD–pH titration experiment was performed. Upon gradual raising the pH of the buffer solution of alkaloid–heparin complexes from 5.7 to 7.9, amplitudes of the ICD bands decreased progressively to zero (Fig. 3 and S2, ESI†). The pH value corresponding to the inflection point of the titration curve gives rise to a pKa of 7.1, which is lower than that reported previously for ellipticine (7.4).7,8 It is worth noting that pKa of the structurally related β-carboline alkaloid norharmane is also 7.1.41 In concordance with the pH dependence of the ICD profile, ellipticine added to pH 7.4 buffer solution of heparin yielded no induced CD signals (Fig. S3, ESI†). In addition, the UV spectroscopic features (band shape, εmax, λmax) are very similar to that obtained in the same, but heparin-free solution (Fig. S4, ESI†). All of these data support that the neutral species of ellipticine does not bind to heparin. It seems that besides H-bonding contribution of the –NH– group, coulombic interaction between the protonated pyridine nitrogen and anionic groups is an additional prerequisite for stabilization of the complexes. In contrast to small molecule–GAG adducts held purely by electrostatic forces,42,43 heparin association of ellipticine is tolerant to the increase of the concentration of highly competitive sodium ions (Fig. S5, ESI†).
Distinctly from heparin and heparan sulfate, ellipticine does not interact with chondroitin 6-sulfate (C6S), the another major GAG component of the extracellular matrix. In relation to that measured in buffer solution, the CD as well as the absorption spectroscopic profile of the alkaloid remains the same in the presence of C6S (Fig. S6, ESI†). This may be attributed to the substantial differences between these GAGs including their primary/secondary structures as well as sulfation patterns (Scheme 2).
Conclusions
Ellipticine, the most studied member of natural pyridocarbazole alkaloids exhibits pH dependent GAG binding properties. Under mildly acidic conditions (pH 6–7), the protonated species are regularly accommodated to the anionic sites of chiral GAG backbones forming a right-handed helical array. This predicts that in acidic malignant as well as inflammatory tissues ellipticine preferentially associates to the extracellular GAG components that are actively involved in a wide variety of signaling cascades modulating cancer and immune cell behavior. Besides DNA intercalation, the GAG affinity of ellipticine demonstrated in the current study helps to better understand its biological actions and may be utilized to develop novel therapeutic interventions targeting neoplastic as well as inflammatory microenvironments.Abbreviations
| C6S | Chondroitin 6-sulfate |
| CD | Circular dichroism |
| GAG | Glycosaminoglycan |
| ICD | Induced circular dichroism |
Acknowledgements
The author is grateful for using the heparan sulfate sample supplied by the Gismo Therapeutics Inc.References
- M. Stiborová and E. Frei, Curr. Med. Chem., 2014, 21, 575–591 CrossRef.
- N. C. Garbett and D. E. Graves, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 149–172 CrossRef CAS PubMed.
- A. Canals, M. Purciolas, J. Aymami and M. Coll, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2005, 61, 1009–1012 CrossRef PubMed.
- S. C. Jain, K. K. Bhandary and H. M. Sobell, J. Mol. Biol., 1979, 135, 813–840 CrossRef CAS PubMed.
- K. W. Kohn, M. J. Waring, D. Glaubiger and C. A. Friedman, Cancer Res., 1975, 35, 71–76 CAS.
- A. Banerjee, S. Sanyal, P. Majumder, P. Chakraborty, K. Jana, C. Das and D. Dasgupta, Biochem. Biophys. Res. Commun., 2015, 462, 352–357 CrossRef CAS PubMed.
- G. Dodin, M. A. Schwaller, J. Aubard and C. Paoletti, Eur. J. Biochem., 1988, 176, 371–376 CrossRef CAS PubMed.
- F. Sureau, F. Moreau, J. M. Millot, M. Manfait, B. Allard, J. Aubard and M. A. Schwaller, Biophys. J., 1993, 65, 1767–1774 CrossRef CAS PubMed.
- Y. Kato, S. Ozawa, C. Miyamoto, Y. Maehata, A. Suzuki, T. Maeda and Y. Baba, Cancer Cell Int., 2013, 13, 89 CrossRef CAS PubMed.
- K. Engin, D. B. Leeper, J. R. Cater, A. J. Thistlethwaite, L. Tupchong and J. D. McFarlane, Int. J. Hyperthermia, 1995, 11, 211–216 CrossRef CAS PubMed.
- E. Iessi, M. L. Marino, F. Lozupone, S. Fais and A. de Milito, Cancer Ther., 2008, 6, 55–66 CAS.
- S. Peppicelli, F. Bianchini and L. Calorini, Cancer Metastasis Rev., 2014, 33, 823–832 CrossRef CAS PubMed.
- T. Yoneda, M. Hiasa, Y. Nagata, T. Okui and F. White, Biochim. Biophys. Acta, 2015, 1848, 2677–2684 CrossRef CAS PubMed.
- S. Y. Choi, C. C. Collins, P. W. Gout and Y. Wang, J. Pathol., 2013, 230, 350–355 CrossRef CAS PubMed.
- K. Rajamaki, T. Nordstrom, K. Nurmi, K. E. Akerman, P. T. Kovanen, K. Oorni and K. K. Eklund, J. Biol. Chem., 2013, 288, 13410–13419 CrossRef CAS PubMed.
- A. E. Greijer, M. C. de Jong, G. L. Scheffer, A. Shvarts, P. J. van Diest and E. van der Wall, Cell. Oncol., 2005, 27, 43–49 CAS.
- L. E. Gerweck, S. Vijayappa and S. Kozin, Mol. Cancer Ther., 2006, 5, 1275–1279 CrossRef CAS PubMed.
- S. M. Bromfield, E. Wilde and D. K. Smith, Chem. Soc. Rev., 2013, 42, 9184–9195 RSC.
- U. Lindahl and L. Kjellen, J. Intern. Med., 2013, 273, 555–571 CrossRef CAS PubMed.
- N. Harris, F. Y. Kogan, G. Il'kova, S. Juhas, O. Lahmy, Y. I. Gregor, J. Koppel, R. Zhuk and P. Gregor, Biochim. Biophys. Acta, 2014, 1840, 245–254 CrossRef CAS PubMed.
- R. V. Iozzo and L. Schaefer, Matrix Biol., 2015, 42, 11–55 CrossRef CAS PubMed.
- J. Halper, Adv. Exp. Med. Biol., 2014, 802, 49–58 CrossRef CAS PubMed.
- I. Fernandez-Vega, O. Garcia-Suarez, B. Garcia, A. Crespo, A. Astudillo and L. M. Quiros, BMC Cancer, 2015, 15, 742 CrossRef PubMed.
- R. Harisi and A. Jeney, OncoTargets Ther., 2015, 8, 1387–1398 Search PubMed.
- Y. M. Coulson-Thomas, T. F. Gesteira, A. L. Norton, W. W. Kao, H. B. Nader and V. J. Coulson-Thomas, Histol. Histopathol., 2015, 30, 33–41 CAS.
- A. D. Theocharis, S. S. Skandalis, G. N. Tzanakakis and N. K. Karamanos, FEBS J., 2010, 277, 3904–3923 CrossRef CAS PubMed.
- F. Zsila, Chirality, 2015, 27, 605–612 CrossRef CAS PubMed.
- F. Zsila, Phys. Chem. Chem. Phys., 2015, 17, 24560–24565 RSC.
- F. Zsila and G. Gedeon, Biochem. Biophys. Res. Commun., 2006, 346, 1267–1274 CrossRef CAS PubMed.
- I. Zegar, A. Graslund, J. Bergman, M. Eriksson and B. Nordén, Chem.-Biol. Interact., 1989, 72, 277–293 CrossRef CAS PubMed.
- G. Behravan, M. Leijon, U. Sehlstedt, B. Norden, H. Vallberg, J. Bergman and A. Graslund, Biopolymers, 1994, 34, 599–609 CrossRef CAS PubMed.
- N. Berova, D. Gargiulo, F. Derguini, K. Nakanishi and N. Harada, J. Am. Chem. Soc., 1993, 115, 4769–4775 CrossRef CAS.
- S. E. Boiadjiev and D. A. Lightner, Monatsh. Chem., 2005, 136, 489–508 CrossRef CAS.
- A. C. Evans, C. Meinert, J. H. Bredehöft, C. Giri, N. C. Jones, S. V. Hoffmann and U. J. Meierhenrich, in Differentiation of Enantiomers II, ed. V. Schurig, Springer International Publishing, 2013, vol. 341, pp. 271–299 Search PubMed.
- M. Monnot, O. Mauffret, V. Simon, E. Lescot, B. Psaume, J. M. Saucier, J. Belehradek Jr and S. Fermandjian, FEBS Lett., 1990, 273, 71–74 CrossRef CAS PubMed.
- K. K. Rohatgi and A. K. Mukhopadhyay, J. Phys. Chem., 1972, 76, 3970–3973 CrossRef CAS.
- M. Weissbluth, Q. Rev. Biophys., 1971, 4, 1–34 CrossRef CAS PubMed.
- S. Krawczyk and R. Luchowski, J. Phys. Chem. B, 2007, 111, 1213–1221 CrossRef CAS PubMed.
- J. Ignacio Tinoco, Annu. Rev. Phys. Chem., 2002, 53, 1–15 CrossRef PubMed.
- A. Delbarre, B. P. Roques, J. B. le Pecq, J. Y. Lallemand and X. Nguyen Dat, Biophys. Chem., 1976, 4, 275–279 CrossRef CAS PubMed.
- O. I. Tarzi and R. Erra-Balsells, J. Photochem. Photobiol., B, 2006, 82, 79–93 CrossRef CAS PubMed.
- L. Zhang, N. Li, F. Zhao and K. Li, Anal. Sci., 2004, 20, 445–450 CrossRef CAS PubMed.
- A. Ziegler and J. Seelig, Biophys. J., 2008, 94, 2142–2149 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and further CD/UV spectroscopic data. See DOI: 10.1039/c5ra23437a |
| This journal is © The Royal Society of Chemistry 2016 |