Effect of TEOS plasma polymerization on corn starch/poly(ε-caprolactone) film: characterization, properties and biodegradation
Abstract
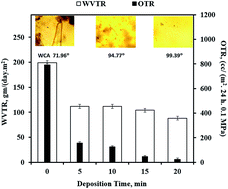
Polymeric packaging materials are preferred because they are lightweight and cost-effective as compared to conventional packaging. Plasma enhanced chemical vapor deposition (PECVD) of organo-silicon compounds is one of the ways to deposit silicon oxide (SiOx) coating on polymers to improve barrier properties. In this paper, a tetraethyl orthosilicate (TEOS) precursor was used to deposit a SiOx coating on corn starch/poly(ε-caprolactone) (CSPCL) films through PECVD. The effect of the deposition time on various properties was studied. ATR-FTIR, XPS and XRD revealed that the coating has a highly cross-linked SiOx glass like structure. AFM and SEM suggested a smooth and conformal morphology. Adhesive properties were studied from the peel strength and correlated with the work of adhesion. Barrier properties were studied from the water vapor and the oxygen transmission rates and showed significant improvement. The effect of the plasma polymerized TEOS (ppTEOS) coating on the biodegradation of CSPCL films was evaluated using an indoor soil burial method (to simulate natural degradation) with the single micro-organism, Bacillus subtilis MTCC 121 (BS 121) (to understand the interaction between micro-organisms & the modified surface). Biodegradation through the indoor soil burial method was assessed by measuring the change in tensile properties and growth of soil micro flora on the surface using optical light microscopy. Biodegradation by BS 121 was assessed by measuring the increase in its number along with the changes it brought about on the sample surface using optical light microscopy and SEM. It was observed that there was a reduction in the adhesion of soil flora and reduced growth of BS121 on ppTEOS coated CSPCL films. Thus ppTEOS coated CSPCL films seem to be an attractive option for environmentally benign packaging applications.

 Please wait while we load your content...
Please wait while we load your content...