The phase evolution, electrical stability and chemical compatibility of sealing glass–ceramics for solid oxide fuel cell applications: effect of La2O3 or CeO2
Honglin Liua,
Xinhang Dua,
Zhiwu Yub,
Dian Tanga and
Teng Zhang*a
aCollege of Materials Science and Engineering, Fuzhou University, Fuzhou, Fujian 350108, China. E-mail: teng_zhang@fzu.edu.cn; Fax: +86 591 22866537; Tel: +86 591 22866540
bHigh Magnetic Field Laboratory, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, Anhui, China
First published on 2nd February 2016
Abstract
In spite of the fact that rare earth oxides can improve the sealing properties of glass–ceramics, the electrical stability of these glass–ceramics under Solid Oxide Fuel Cell (SOFC) operational conditions still remains ambiguous. In this work, the electrical stability of glass–ceramics doped with La2O3 or CeO2 under SOFC operational conditions has been systematically investigated. The glass–ceramic material with La2O3 dopant exhibits good electrical stability under SOFC operational conditions; whereas, a decrease in the conductivity of the CeO2-containing glass–ceramic material can be related to the formation of a conductive phase, i.e., CeO2. In particular, the relationship between the phase evolution and the change in conductivity of glass–ceramics has been clearly demonstrated. Moreover, the sealing glass–ceramics show good chemical compatibility with 8 mol% yttria-stabilized zirconia (8YSZ) electrolyte, after being held at 750 °C for 1000 hours. The reported results support the suitability of La2O3-containing glass–ceramic as a sealing material for SOFC applications.
1. Introduction
The direct operation of Solid Oxide Fuel Cells (SOFCs) on hydrocarbon fuels has attracted increasing attention in the last decade, due to the large amount of hydrocarbons as well as SOFCs showing the highest conversion efficiency among all types of fuel cells. Planar SOFCs have become increasingly attractive, due to their lower cost and higher power density per unit volume compared with tubular designs.1–5 However, there are many edges that need to be sealed at high temperature. Therefore, sealing materials are of great importance for planar SOFC components. Sealing glasses and glass–ceramics have been proven to be the most promising candidates, and thus constitute a very active field of research.6–10To accomplish the sealing targets of planar SOFCs, many requirements for sealing materials need to be fulfilled, including sealing properties, thermal stability, thermo-mechanical stability, and electrical stability, as well as chemical compatibility.11–14 To reduce the coefficient of thermal expansion (CTE) mismatch between sealing glass–ceramics and other SOFC components, network modifiers, e.g., alkaline earth metal oxides15,16 and alkaline metal oxides,17,18 are often included in the sealing materials. However, the presence of such mobile ions contributes to an increase in electrical conductivity of the glasses and glass–ceramics,19,20 and thus, imposes additional uncertainty on the electrical insulation of the sealing glass–ceramics, especially under the long-term operation of a SOFC. For example, Huang et al.21 reported a Y2O3–BaO–SiO2–B2O3–Al2O3 glass sealant with a CTE of 11.64 K−1 between 323 and 873 K, which falls in the desired CTE range for SOFC application (9–12 × 10−6 K−1).16,22 However, its conductivity was about 5 × 10−6 S cm−1 at 750 °C, mainly due to the high BaO content in the glass (60 wt%). In addition, the conductivity of a SrO–La2O3–Al2O3–B2O3–SiO2 glass developed by Ojha et al. ranged from 2.70 × 10−6 to 5.68 × 10−7 S cm−1 in the temperature range of 600–800 °C.23 Therefore, the electrical properties of sealing glass–ceramics have attracted increasing attention in recent years.6,24–27
On the other hand, rare earth oxides such as La2O3 and CeO2 have been proven to be beneficial to the sintering properties of glass–ceramics,22,28 which is highly desirable in SOFC sealing applications. However, the effect of rare earth oxides on the electrical stability of glass–ceramics under SOFC operational conditions still remains ambiguous, and needs to be clarified before they can be seriously considered as sealing candidates for SOFC applications.
In this paper, La2O3 or CeO2 was added into a representative borosilicate glass system. Attention was focused on the effect of La2O3 or CeO2 on the electrical stability of glass–ceramics under SOFC operational conditions. In particular, the relationship between the phase evolution and the change in conductivity of glass–ceramics was established to provide useful information for the development of reliable sealing materials for SOFC applications.
2. Experimental
A 50 g sample of glass designated ‘GA’ was prepared from a batch mixture of reagent grade alkaline earth carbonates, boric acid, and various oxides to form the nominal glass composition (mol%): 25.0CaO–25.0SrO–10.0Al2O3–7B2O3–33.0SiO2. Glasses doped with La2O3 or CeO2 were also prepared with nominal glass compositions (mol%) of 24.5CaO–24.5SrO–9.8Al2O3–6.9B2O3–32.3SiO2–2La2O3 and 24.0CaO–24.0SrO–9.6Al2O3–6.7B2O3–31.7SiO2–4CeO2, respectively. The sealing glasses with La2O3 or CeO2 dopant are designated as glass#GL and glass#GC, respectively. The mole fractions of La or Ce ions in the glasses are designed to be 4 mol% for comparison. The batches were melted in a platinum crucible at a temperature of 1400–1500 °C for 2 hours in air. Some of the melt was poured into a stainless steel mold to obtain cylindrical shaped glass specimens (25 mm length and 10 mm diameter) and the rest of the melt was quenched on a steel plate. Glass powders were then crushed and sieved to particle sizes of 45 to 53 μm.Pellets (10 mm diameter and 2 mm thickness) were formed via uniaxial pressing of the glass powders before heating at 750 °C for up to 1000 h. A high resistance meter (4339B, Agilent, Inc.) was used to measure the conductivity of the glasses and glass–ceramic pellets in air from 600 to 750 °C.
The coefficient of thermal expansion (CTE, between 200 and 600 °C), glass transition temperature (Tg) and softening temperature (Td) of the quenched glasses were determined using a dilatometer (DIL402C, NETZSCH, Inc.) at 10 °C min−1 in air. The glass–ceramic samples were held at 750 °C for 1000 hours and were also subjected to CTE measurements for comparison. The thermal properties of the glasses and glass–ceramics, including Tg, Td and CTE, are summarized in Table 1.
| Sample ID | Glass#GA | Glass#GL | Glass#GC |
|---|---|---|---|
| Thermal parameters (°C) | |||
| Tg | 712 ± 5 | 726 ± 5 | 727 ± 5 |
| Td | 769 ± 5 | 864 ± 5 | 773 ± 5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| CTE (200–600 °C) × 10−6 (K−1) | |||
| Glasses | 13.3 ± 0.1 | 10.7 ± 0.1 | 9.9 ± 0.1 |
| Glass–ceramics | 9.7 ± 0.1 | 9.7 ± 0.1 | 9.2 ± 0.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Density (g cm−3) | |||
| Glasses | 3.24 ± 0.01 | 3.33 ± 0.01 | 3.29 ± 0.01 |
| Glass–ceramics | |||
| 750 °C 100 h | 3.08 ± 0.01 | 3.28 ± 0.01 | 3.35 ± 0.01 |
| 750 °C 500 h | 3.18 ± 0.01 | 3.26 ± 0.01 | 3.34 ± 0.01 |
| 750 °C 1000 h | 3.11 ± 0.01 | 3.28 ± 0.01 | 3.33 ± 0.01 |
The crystalline phases in the glass–ceramic samples were identified via X-ray diffraction (XRD, XDS 2000, Scintag, Inc.). The relative content of crystalline phases (in wt%) in CeO2-containing species was then calculated using RIQAS software (Release 4.0.0.8, Materials Data, Inc., CA).
The glass–ceramic pellets were polished using SiC paper ranging from 320 to 1200 grit, and were then finished using an alumina suspension (3 μm). The polished samples were analyzed using field emission scanning electron microscopy (Supra-55, Zeiss, Inc.) and energy dispersive analysis by X-rays (X-Max, OXFORD instruments, Inc.). The glasses were bonded to an 8 mol% YSZ (8YSZ) electrolyte (AR, Sinopharm Chemical Reagent Co., Ltd., China) and their interfacial reactions were characterized. Glass pastes were prepared by mixing ∼50 mg of the glass powder (particle size of 45–53 μm) with ∼50 μL of acetone. The pastes were applied to the 8YSZ surfaces after ultrasonic cleaning. The coatings were subsequently heated in air at 750 °C for 1000 h. Cross sections of the glass/8YSZ sealing couples were polished and analyzed using FE-SEM and EDS.
3. Results and discussion
Fig. 1a shows the conductivity of the glasses measured in air from 600 to 750 °C. It is clear that the conductivity of the glasses increases with increasing temperature. This indicates that ionic conduction is the main mechanism in the present work.29,30 In addition, the conductivity of the glasses decreases with the addition of La2O3 or CeO2. For example, the conductivity of the glasses at 750 °C decreases from 8.05 × 10−7 S cm−1 for glass#GA to 2.63 × 10−7 S cm−1 for glass#GC and to 1.90 × 10−7 S cm−1 for glass#GL. It is worth noting that the conductivity of the La2O3-containing glass–ceramics is about two orders of magnitude lower than that of the SrO–La2O3–Al2O3–B2O3–SiO2 glass under similar conditions (e.g., 1.03 × 10−8 vs. 2.70 × 10−6 S cm−1, measured at 600 °C).23 The Arrhenius plots in Fig. 1a have been fitted to calculate the activation energy of ionic conduction in glass. The similar activation energies, ranging from 145 ± 10 to 160 ± 10 kJ mol−1, confirm the same conductive mechanism in all of the glasses.Fig. 1b shows the conductivity of the glass–ceramics held at 750 °C for 1000 h. The conductivity of the glass–ceramics increases with increasing temperature, exhibiting the characteristics of ionic conduction. It is clear that the conductivity of glass#GA and glass#GL is much lower than that of glass#GC. In addition, the activation energy of ionic conduction in the glass–ceramics increases from 124 ± 10 kJ mol−1 for glass#GC to 150 ± 10 kJ mol−1 for glass#GA.
To investigate the electrical stability of the glass–ceramics under a SOFC operational environment, the conductivity of the glass–ceramics measured at 750 °C is plotted as a function of the heat-treatment time, as shown in Fig. 1c. The conductivity of glass#GA and glass#GL decreases slightly with increasing time and reaches a constant after 500 h; whereas, the conductivity of glass#GC increases by an order of magnitude in the first 100 h, and then decreases to a constant after 500 h. For example, the conductivity of glass#GL after being held at 750 °C for 24, 100, 500, and 1000 h is 1.00 × 10−7, 6.70 × 10−8, 3.38 × 10−8, and 4.16 × 10−8 S cm−1, respectively. In contrast, the conductivity of glass#GC after being held at 750 °C for 24, 100, 500, and 1000 h is 5.92 × 10−8, 5.21 × 10−7, 1.54 × 10−7, and 1.30 × 10−7 S cm−1, respectively. It is also worth noting that the conductivity of the glasses and glass–ceramics in the present work is much lower than in the reported results for sealing glass–ceramics in the literature.7
Table 1 shows the thermal properties of the glasses and glass–ceramics. It is clear that the glass transition temperature (Tg) and softening temperature (Td) increase with the introduction of the La2O3 or CeO2 dopants. In addition, the coefficient of thermal expansion (CTE) of the glasses decreases with the addition of La2O3 or CeO2. The increase in Tg and Td, as well as the decrease in CTE, indicates that a condensed glass network is formed by the La2O3 or CeO2 dopants.31 Moreover, the density of glass#GA is lower than that of glass#GL and glass#GC, which can be related to their strengthened glass structure, as well as the larger atomic weights of La and Ce compared with the other elements in the glass. The condensed glass structure contributes to the decrease in conductivity of the glass (Fig. 1a), due to the enhanced restriction of ionic diffusion.27,30,32 On the other hand, the density of the glass–ceramics after being held at 750 °C does not change significantly with heat-treatment time, which excludes the effect of the glass matrix on the conductivity of the glass–ceramics.27 The CTE of the glass–ceramics ranges from 9.2 to 9.7 × 10−6 K−1, which is within the designed CTE range for sealing glass–ceramics.22
Fig. 2a shows the XRD patterns of glass#GA held at 750 °C for up to 1000 h. The main phases in the glass–ceramics are Ca2Al2SiO7 (ICDD Card No. 73-2041), SrAl2Si2O8 (ICDD Card No. 70-1862), Ca2SiO4 (ICDD Card No. 72-1660), Sr2SiO4 (ICDD Card No. 76-1494), and Ca2B2O5 (ICDD Card No. 79-1516). Fig. 2b shows the XRD patterns of glass#GL held at 750 °C for up to 1000 h. In addition to the phases present in glass#GA, a new La-containing phase, i.e., La5SiBO13 (ICDD Card No. 52-0699), can be observed in glass#GL. The XRD patterns in Fig. 2a and b do not change significantly with time, implying the phase stability of glass#GA and glass#GL. Based on the similar densities of the glass–ceramics (Table 1) and the phase stability (Fig. 2) of the glass–ceramics, one can conclude that the decrease in conductivity of glass#GA and glass#GL with increasing time is mainly attributed to crystal growth in the glass–ceramics, which reduces the number of grain boundaries and consequently decreases the most effective pathways for ionic diffusion.33,34
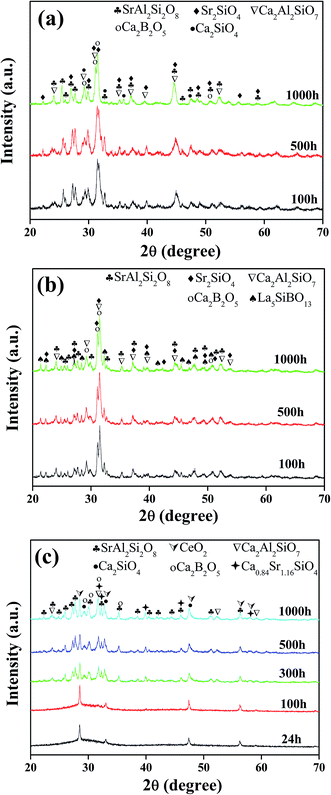 | ||
| Fig. 2 XRD patterns of the glass–ceramics held at 750 °C. (a) Glass#GA, (b) glass#GL, and (c) glass#GC. | ||
Shown in Fig. 2c are the XRD patterns of glass#GC held at 750 °C for up to 1000 h. It is clear that CeO2 (ICDD Card No. 81-0792) is the only phase detected in the glass–ceramic material held at 750 °C for up to 100 h; whereas, some more phases can be identified in the specimen after more than 100 h, including Ca2Al2SiO7, SrAl2Si2O8, Ca2SiO4, Ca2B2O5 and Ca0.84Sr1.16SiO4 (ICDD Card No. 77-0474). Table 2 summarizes the quantitative XRD results for glass#GC held at 750 °C for up to 1000 h. In addition, the calculated crystalline content in the CeO2-containing glass–ceramics increases from 2 wt% at 24 h to 5 wt% at 100 h. Therefore, the increase in conductivity of glass#GC from 5.92 × 10−8 S cm−1 at 24 h to 5.21 × 10−7 S cm−1 at 100 h relates to the increasing CeO2 content in the glass–ceramic material, due to its good conductivity under SOFC operational conditions.35 Moreover, the decrease in conductivity of glass#GC from 5.21 × 10−7 S cm−1 at 100 h to 1.30 × 10−7 S cm−1 at 1000 h can be ascribed to the formation of insulative phases, such as Ca2Al2SiO7, SrAl2Si2O8, Ca2SiO4, Ca0.84Sr1.16SiO4 and Ca2B2O5.36–38
| Time | CeO2 | Ca0.84Sr1.16SiO4 | Ca2Al2SiO7 | Ca2SiO4 | SrAl2Si2O8 | Ca2B2O5 |
|---|---|---|---|---|---|---|
| 24 h | 100 | |||||
| 48 h | 100 | |||||
| 100 h | 100 | |||||
| 300 h | 6 | 17 | 13 | 4 | 36 | 24 |
| 500 h | 6 | 17 | 15 | 4 | 35 | 24 |
| 1000 h | 5 | 17 | 20 | 6 | 33 | 19 |
Fig. 3 shows the SEM micrographs of glass#GA held at 750 °C for up to 1000 h. The gray regions are found to contain a significant amount of Sr, Al and Si, confirming the formation of SrAl2Si2O8 in glass#GA. In addition, the crystal growth can be observed by comparing the size of the gray regions in Fig. 3a and b. This is in good agreement with the decrease in conductivity of glass#GA with increasing time (Fig. 1).
Fig. 4 shows the SEM micrographs of glass#GL held at 750 °C for up to 1000 h. The La-enrichment can be observed in the white regions, in agreement with the formation of La5SiBO13 in the XRD results (Fig. 2). The crystal growth of La5SiBO13 can also be observed with increasing time, which contributes to the decrease in conductivity of glass#GL with increasing time (Fig. 1).
Shown in Fig. 5 are the SEM micrographs of glass#GC held at 750 °C for up to 1000 h. It is clear that some small crystals are present in the glass–ceramic material at 24 and 100 h (Fig. 5a and b), which can be identified as CeO2. In addition, crystalline CeO2 is scattered in the glass–ceramic material at 500 and 1000 h (Fig. 5c and d). This further verifies that the decrease in conductivity of glass#GC from 100 h to 1000 h results from the formation of insulative phases.
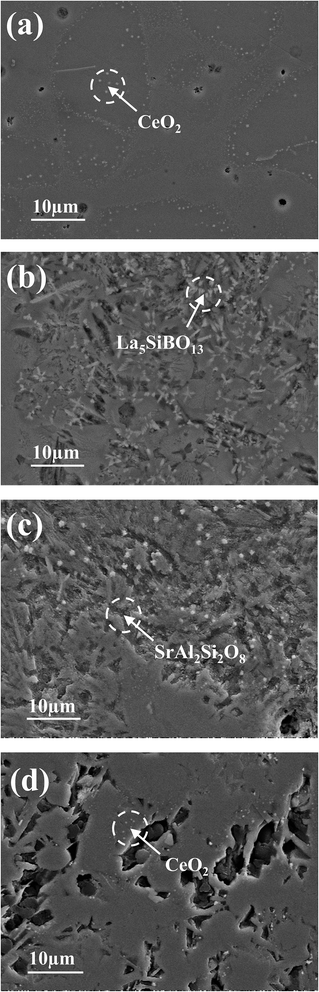 | ||
| Fig. 5 SEM images of glass#GC crystallized at 750 °C for (a) 24 h, (b) 100 h, (c) 500 h, and (d) 1000 h. | ||
Fig. 6 shows SEM micrographs of the glass–ceramic/8YSZ sealing couples held at 750 °C for 1000 h, along with elemental EDS line scans taken across the interface. The EDS line scans reveal the formation of an interdiffusion zone with a thickness of about 4 μm at the glass#GA/8YSZ interface (Fig. 6a); whereas, the thickness of the interdiffusion zone at the interface between glass#GL or glass#GC and 8YSZ is about 2 μm (Fig. 6b and c). This indicates that the interdiffusion between the glass and 8YSZ electrolyte can be effectively reduced by the addition of La2O3 or CeO2. In addition, all of the sealing couples remain intact after holding at 750 °C for 1000 h, which can be mainly related to the close CTE between the glass–ceramics and 8YSZ. The considerable amount of dendrites in the glass–ceramics, e.g., SrAl2Si2O8 in all species (Fig. 3–5) and La5SiBO13 in glass#GL (Fig. 4), also contribute to the good sealing performance under SOFC operational conditions.39
 | ||
| Fig. 6 SEM micrographs of the glass/8YSZ sealing couples held at 750 °C for 1000 h, along with the elemental EDS line scans taken across the interface. (a) Glass#GA, (b) glass#GL, and (c) glass#GC. | ||
4. Conclusions
The effect of La2O3 or CeO2 on the phase evolution, electrical stability and chemical compatibility of sealing glass–ceramics has been investigated in this work. The addition of La2O3 or CeO2 condenses the glass network and decreases the conductivity of the glass. In addition, the La2O3-containing glass–ceramic material exhibits good electrical stability under SOFC operational conditions. In contrast, the formation of CeO2 in CeO2-doped glass–ceramic leads to a decrease in electrical stability. Moreover, the interdiffusion between glass and 8YSZ electrolyte can be effectively reduced by the addition of La2O3 or CeO2. The findings on the phase evolution, electrical stability and chemical compatibility of glass–ceramics doped with rare earth oxide will shed light onto the design of stable sealing materials for SOFC applications.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 51102045 and No. 21203202), and Program for New Century Excellent Talents in Fujian Province University (No. JA12013). They would also like to thank Zhenhuan Zheng for assistance with SEM/EDS and X-ray diffraction experiments, and Fen Lin for assistance with dilatometric measurements.References
- N. Mahato, A. Banerjee, A. Gupta, S. Omar and K. Balani, Prog. Mater. Sci., 2015, 72, 141–337 CrossRef CAS.
- K. Wang, D. Hissel, M. C. Péra, N. Steiner, D. Marra, M. Sorrentino, C. Pianese, M. Monteverde, P. Cardone and J. Saarinen, Int. J. Hydrogen Energy, 2011, 36, 7212–7228 CrossRef CAS.
- X. Zhang, S. H. Chan, G. Li, H. K. Ho, J. Li and Z. Feng, J. Power Sources, 2010, 195, 685–702 CrossRef CAS.
- A. Choudhury, H. Chandra and A. Arora, Renewable Sustainable Energy Rev., 2013, 20, 430–442 CrossRef CAS.
- A. B. Stambouli and E. Traversa, Renewable Sustainable Energy Rev., 2002, 6, 433–455 CrossRef CAS.
- Y. S. Chou, J. W. Stevenson, G. G. Xia and Z. G. Yang, J. Power Sources, 2010, 195, 5666–5673 CrossRef CAS.
- S. Ghosh, A. Das Sharma, P. Kundu, S. Mahanty and R. N. Basu, J. Non-Cryst. Solids, 2008, 354, 4081–4088 CrossRef CAS.
- V. Kumar, G. Kaur, K. Lu and G. Pickrell, Int. J. Hydrogen Energy, 2015, 40, 1195–1202 CrossRef CAS.
- J. H. Hsu, C. W. Kim and R. K. Brow, J. Power Sources, 2014, 250, 236–241 CrossRef CAS.
- M. J. Pascual, A. Guillet and A. Durán, J. Power Sources, 2007, 169, 40–46 CrossRef CAS.
- I. W. Donald, P. M. Mallinson, B. L. Metcalfe, L. A. Gerrard and J. A. Fernie, J. Mater. Sci., 2011, 46, 1975–2000 CrossRef CAS.
- M. K. Mahapatra and K. Lu, J. Power Sources, 2010, 195, 7129–7139 CrossRef CAS.
- M. K. Mahapatra and K. Lu, Mater. Sci. Eng., R, 2010, 67, 65–85 CrossRef.
- D. U. Tulyaganov, A. A. Reddy, V. V. Kharton and J. M. F. Ferreira, J. Power Sources, 2013, 242, 486–502 CrossRef CAS.
- S. Sakuragi, Y. Funahashi, T. Suzuki, Y. Fujishiro and M. Awano, J. Power Sources, 2008, 185, 1311–1314 CrossRef CAS.
- S. E. Lin, Y. R. Cheng and W. C. J. Wei, J. Eur. Ceram. Soc., 2011, 31, 1975–1985 CrossRef CAS.
- Q. Zhang, Y. Zhu and Z. Li, J. Non-Cryst. Solids, 2012, 358, 680–686 CrossRef CAS.
- C. Ritzberger, E. Apel, V. M. Rheinberger, M. Schweiger and W. Hoeland, Phys. Chem. Glasses, 2013, 54, 228–231 CAS.
- A. Grandjean, M. Malki, V. Montouillout, F. Debruycker and D. Massiot, J. Non-Cryst. Solids, 2008, 354, 1664–1670 CrossRef CAS.
- A. Grandjean, M. Malki and C. Simonnet, J. Non-Cryst. Solids, 2006, 352, 2731–2736 CrossRef CAS.
- S. Huang, Q. Lu and C. Wang, J. Alloys Compd., 2011, 509, 4348–4351 CrossRef CAS.
- A. Goel, A. A. Reddy, M. J. Pascual, L. Gremillard, A. Malchere and J. M. F. Ferreira, J. Mater. Chem., 2012, 22, 10042 RSC.
- P. K. Ojha, T. K. Chongdar, N. M. Gokhale and A. R. Kulkarni, Int. J. Hydrogen Energy, 2011, 36, 14996–15001 CrossRef CAS.
- A. Dutta, T. P. Sinha, P. Jena and S. Adak, J. Non-Cryst. Solids, 2008, 354, 3952–3957 CrossRef CAS.
- M. L. Braunger, C. A. Escanhoela Jr, I. Fier, L. Walmsley and E. C. Ziemath, J. Non-Cryst. Solids, 2012, 358, 2855–2861 CrossRef CAS.
- J. Chen, Q. Zou, F. Zeng, S. Wang, D. Tang, H. Yang and T. Zhang, J. Power Sources, 2013, 241, 578–582 CrossRef CAS.
- H. Liu, D. Zhao, S. Chen, J. Huang, D. Tang and T. Zhang, RSC Adv., 2015, 5, 62891–62898 RSC.
- S. B. Sohn and S.-Y. Choi, J. Non-Cryst. Solids, 2001, 282, 221–227 CrossRef CAS.
- C. H. Hsieh and H. Jain, J. Non-Cryst. Solids, 1995, 183, 1–11 CrossRef CAS.
- S. Chen, Z. Yu, Q. Zhang, J. Wang, T. Zhang and J. Wang, J. Eur. Ceram. Soc., 2015, 35, 2427–2431 CrossRef CAS.
- Y. H. Zhang, A. Navrotsky, H. Li, L. Y. Li, L. L. Davis and D. M. Strachan, J. Non-Cryst. Solids, 2001, 296, 93–101 CrossRef CAS.
- V. K. Deshpande and R. N. Taikar, Mater. Sci. Eng., B, 2010, 172, 6–8 CrossRef CAS.
- A. C. S. Sabioni, J. N. V. Souza, V. Ji, F. Jomard, V. B. Trindade and J. F. Carneiro, Solid State Ionics, 2015, 276, 1–8 CrossRef CAS.
- K. Marquardt, E. Petrishcheva, E. Gardés, R. Wirth, R. Abart and W. Heinrich, Contrib. Mineral. Petrol., 2011, 162, 739–749 CrossRef CAS.
- M. S. H. Tinku Baidya and J. Gopalakrishnan, J. Phys. Chem. B, 2007, 111, 5149–5154 CrossRef PubMed.
- H. Takeda, M. Hagiwara, H. Noguchi, T. Hoshina, T. Takahashi, N. Kodama and T. Tsurumi, Appl. Phys. Lett., 2013, 102, 242907 CrossRef.
- C. C. Chiang, S. F. Wang, Y. R. Wang and Y. F. Hsu, J. Alloys Compd., 2008, 461, 612–616 CrossRef CAS.
- X. C. Wang, W. Lei, R. Ang and W. Z. Lu, Ceram. Int., 2013, 39, 1707–1710 CrossRef CAS.
- L. H. Fang, Q. Zhang, F. Lin, D. Tang and T. Zhang, J. Eur. Ceram. Soc., 2015, 35, 2201–2207 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



