Synthesis, characterization and fabrication of ultrathin iron pyrite (FeS2) thin films and field-effect transistors†
Abstract
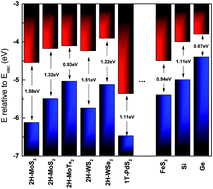
We reported a synthesis of an ultrathin FeS2 thin film via thermal sulfurization of an iron thin film and its fabrication process for field-effect transistors. Reaction time was found to be essential for the growth of the crystallized FeS2 thin film. The thickness of Fe increased from 10 nm to 35 nm after the reaction. The FeS2 thin film has a resistivity of 0.345 Ω cm and a Hall mobility of 7.1 cm2 V−1 s−1, with a carrier concentration of 2.89 × 1018 cm−3. The fabricated FeS2 transistors have a reported highest current on/off ratio of 3.94 × 104 and Ion of 0.117 mA. Moreover, the FeS2 based transistor not only broader the applications of pyrite, but also provides a platform for investigation of FeS2 materials. Temperature-dependent electrical transport measurements confirmed the rich intrinsic defect states in the FeS2 thin film, which indicates that reducing intrinsic defect might be the key issue to further improve the device performance.

 Please wait while we load your content...
Please wait while we load your content...