Anti-Malassezia furfur activity of natural surfactant mediated in situ silver nanoparticles for a better antidandruff shampoo formulation†
Abstract
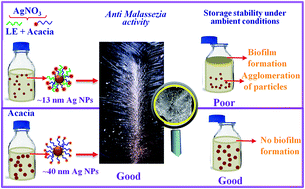
Dandruff is a common scalp problem of human beings and a majority of people suffer from this at some point in their life. To prevent dandruff, antidandruff shampoos have become popular in recent years. However, in many cases, a dandruff causing fungus, Malassezia furfur, develops resistance towards commonly used antidandruff drugs. As a result, it is necessary to develop a new class of novel antidandruff shampoos for dual purposes. This study mainly focuses on a silver nanoparticle based green formulation of an antidandruff shampoo. In situ capped silver nanoparticles (Ag NPs) were prepared by green routes using Acacia and Acacia + Aegle marmelos leaf extracts (LEs) with sizes of ∼40 and 13 nm, respectively. The antifungal activity tests using a well diffusion technique show that 13 nm particles have better inhibiting activity (2.43 times) than 40 nm particles, while the TTC assay method shows that the minimum or threshold concentration is the same for both particle sizes used, which is 0.054 mM. The time kill assay shows some synergism in the net antifungal effect by Acacia + LEs and ∼13 nm Ag NPs in aqueous media against M. furfur. However, the promising anti-Malassezia activity of the 40 nm Ag NPs in the Acacia media along with their superior suspension stability against microbial contamination mean they have potential as an active and simple antidandruff shampoo formulation.


 Please wait while we load your content...
Please wait while we load your content...