Proton gated oxide electric-double-layer transistors for full-swing low voltage inverter applications
Abstract
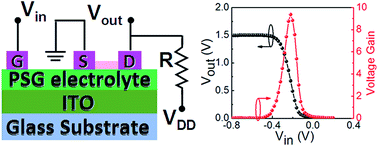
Ion gated electric-double-layer (EDL) transistors provide promising strategies to achieve electrostatic modulation for solid-state materials. Here, proton gated indium-zinc-oxide (IZO) EDL transistors were fabricated by using nanogranular phosphorosilicate glass (PSG) based electrolytes as gate dielectrics. A high proton conductivity of ∼3.9 × 10−4 S cm−1 and an extremely big EDL capacitance of ∼4.4 μF cm−2 were observed for the PSG based electrolyte. The IZO EDL transistors could operate at a low voltage of 1.5 V. Furthermore, resistor-loaded inverters were built, demonstrating modulatable electrical performances by a modulatable operation mode of the driving IZO transistor. Full-swing inverter characteristics were observed at a low supply voltage of 1.5 V, exhibiting a high voltage gain of above 7. The inverters were also used for decreasing the noises of input signals. The proton gated oxide EDL transistors have potential applications in biochemical sensors and portable electronics.

 Please wait while we load your content...
Please wait while we load your content...