Hexagonal microspindle of NH2-MIL-101(Fe) metal–organic frameworks with visible-light-induced photocatalytic activity for the degradation of toluene†
Zhiguang Zhangab,
Xinyong Li*a,
Baojun Liua,
Qidong Zhaoa and
Guohua Chen*c
aKey Laboratory of Industrial Ecology and Environmental Engineering, School of Environmental Science & Technology, Dalian University of Technology, Dalian 116024, China. E-mail: xinyongli@hotmail.com; xyli@dlut.edu.cn; Fax: +86-411-8470-7733; Tel: +86-411-8470-7733
bSchool of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian, Liaoning 116029, China
cDepartment of Chemical and Biomolecular Engineering, The Hong Kong University of Science &Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: kechengh@ust.hk; Fax: +852-2358-0054; Tel: +852-2358-7138
First published on 22nd December 2015
Abstract
This work focuses on exploring metal–organic frameworks (MOFs) for degradation of gaseous pollutants. We demonstrate that NH2-MIL-101(Fe) hexagonal micro-spindles, as a new photocatalyst, showed an improved performance for degradation of toluene under visible light irradiation. The structural and optical properties of the as-prepared NH2-MIL-101(Fe) hexagonal micro-spindles were characterized. Furthermore, the catalytic reaction mechanism has been investigated on the basis of in situ Fourier Transform infrared spectroscopy (FTIR) technology. Meanwhile, some intermediates (benzoic acid) and the final product (CO2) of the degradation of toluene were also identified.
1. Introduction
Metal–organic frameworks (MOFs) are crystalline porous hybrid materials made by multifunctional organic molecular building blocks and metal or metal cluster connecting nodes.1 Because of their well-ordered porous structures, large surface area, highly diversified framework topologies and tunable organic functionality, MOFs exhibit many potential applications, such as gas separation and adsorption,2–4 hydrogen storage,5,6 catalysis,7,8 and molecular recognition.9 Especially in the field of photocatalysis, organic linkers could act as antennas to absorb light, activate metal clusters, and form organic-linker-to-metal-cluster charge transfer (LCCT),10 leading to a lower charge recombination rate of photoinduced electron–hole pairs.11 More importantly, tunable organic functionality, such as excellent optical properties and strong absorption of visible light could be obtained by simple modifying the organic linkers of MOFs.12 Recently, it has been proved that MOFs, as alternative photocatalysts, show attractive performances in hydrogen production,13 reduction of CO2,14 and degradation of dye,15 etc. In addition, Fe-based MOF materials have been reported and exhibit large absorption coefficient in the region of visible light due to the existence of iron oxo-clusters. Some groups demonstrate the Fe3-μ3-oxo clusters with small sizes could improve mobility of photo-induced carriers and thus suppress recombination of electron–hole pairs. Furthermore, visible-light response range could be tuned by substitution of amino group for organic linkers and variation of morphologies due to quantum confinement effect.16 Therefore, more attentions have been paid to preparation of nanostructured Fe(III)-MOFs (MIL-88A, MIL-53) as well as their applications in photocatalytic degradation of aqueous pollutants.17–19Volatile organic compounds (VOCs) are a class of primary gaseous pollutants in atmosphere and indoor environment, which have raised wide attention on their greenhouse effect and photochemical smog in the environment.20–22 Hence, the development of degradation methods for these pollutants is in urgent demand. Compared with physical, chemical-adsorption and biological methods, photocatalysis technique has been widely investigated due to its cost-effective and environmentally friendly superiorities.23–25 Up to now, various photocatalysts, including inorganic and organic–inorganic semiconductors, have been prepared and simultaneously applied to photodegradation of liquid and gaseous pollutants.26–29 However, photocatalytic degradation of VOCs over MOFs is rarely reported, in spite of their better adsorption to gas, which could be beneficial to achieving quick mass diffusion.
In this study, we successfully fabricated hexagonal microspindle-type MOFs photocatalyst of NH2-MIL-101(Fe) for degradation of gaseous toluene (a typical component of VOCs) under visible light illumination. Owing to its inherent absorbance of visible light and efficiently separated from one another in a metal–organic framework, NH2-MIL-101(Fe) is a good candidate for the photocatalytic degradation of gaseous pollutants.30 Furthermore, the photocatalytic degradation mechanism of NH2-MIL-101(Fe) was investigated in details by in situ Fourier transform infrared spectroscopy (FTIR).
2. Experimental section
2.1 Preparation of the NH2-MIL-101(Fe) hexagonal microspindle
All the reagents and solvents were commercially available and used as received. NH2-MIL-101(Fe) was prepared by following previously reported procedures with some modifications.31 Typically, FeCl3·6H2O and 2-aminoterephthalic acid (H2ATA) were dissolved in DMF solution (the molar ratio was set to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156), and then the mixed solution was put into a 100 mL Teflon-lined autoclave at 120 °C for 24 h. After the hydrothermal processing, the brown powder was collected and repeatedly washed with DMF and methanol, and then dried at 60 °C overnight.
156), and then the mixed solution was put into a 100 mL Teflon-lined autoclave at 120 °C for 24 h. After the hydrothermal processing, the brown powder was collected and repeatedly washed with DMF and methanol, and then dried at 60 °C overnight.
2.2 Characterizations
The morphology of the NH2-MIL-101(Fe) was characterized by using field emission scanning electron microscope (FE-SEM, HITACHI SU-8010), and the elements were verified by energy dispersive X-ray (EDX) spectroscopy detector, equipped with the FE-SEM above. Transmission electron microscopy (TEM) analyses were carried out on a JEOL-2010 electron microscope, using a 200 kV accelerating voltage. The X-ray diffraction (XRD) data for crystalline structure of the prepared samples were obtained from 5° to 30°. Fourier transform infrared spectra (FTIR, BRUKER VERTEX 70) were recorded in the range 4000–450 cm−1 with 4 cm−1 resolution. Simple FTIR collection was conducted at room temperature in the open environment without chamber, when the pellet of sample prepared with KBr was fixed vertically on the holder of the standard accessory accompanied with instrument. The surface properties and composition are conducted by X-ray photoelectron spectroscopy (XPS, ESCA Lab 250, VG Scientific Ltd.). The UV-vis absorption spectra were measured using UV-vis absorption spectrophotometer (JASCO, UV-550) in the spectral range of 200–800 nm.2.3 Photocatalytic activity test
Photocatalytic activity of the NH2-MIL-101(Fe) hexagonal microspindle was evaluated by conversion of toluene under visible light in a home-made in situ quartz infrared reaction cell.32 The sample piece (0.02 g) was set in the sample holder, and then a volume of 4 μL liquid toluene was injected into the sealed reaction cell using a micro syringe. After an hour in the dark, the gaseous toluene would reach adsorption equilibrium on the surface of the catalyst prepared. At that time, the amount of toluene was designated as the initial concentration. As light resource, a 500 W xenon lamp with a UV-cutoff filter (λ > 400 nm) (Shanghai Lansheng Electronic Co., Ltd.) was turned on, and the toluene concentration for photocatalytic degradation was calculated by the integral area in the region of 3100–2900 cm−1 for characteristic peaks of toluene. The reactor temperature is controlled as about 22–25 °C. During the reaction process, the reaction mechanisms and intermediates were studied through FTIR spectrometer (Bruker VERTEX 70).3. Results and discussion
3.1 The chemical and physical properties of NH2-MIL-101(Fe)
X-ray powder pattern of the NH2-MIL-101(Fe) hexagonal microspindle is shown in Fig. 1a. The main diffraction peaks of the as-prepared samples are similar to that of the previously reported pattern of MIL-101 family.33,34 By referring to the fact of the standard, here, the 3D crystal model has been used to simulate XRD pattern. Meanwhile, the XRD patterns of the synthesized samples exhibited flat background and strong intensity, indicating the high crystallinity of the samples. Moreover, for the as-obtained product, no other peaks were detected, indicating that pure phase of NH2-MIL-101(Fe) has been obtained.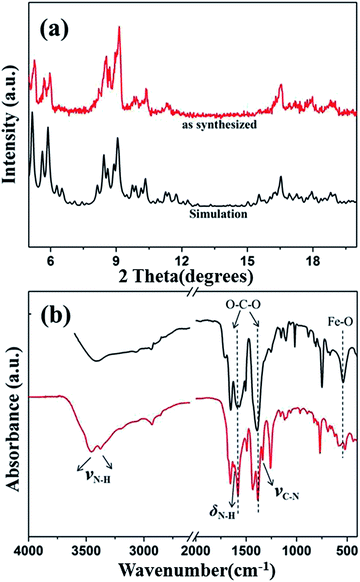 | ||
| Fig. 1 XRD patterns (a) and FTIR spectra (b) of the NH2-MIL-101(Fe) (red) and MIL-101(Fe) (black) samples. | ||
In order to further analyze the molecular structure and identify the functional groups of NH2-MIL-101(Fe) sample, the FTIR spectroscopy was obtained and the results are shown in Fig. 1b. Strong bands at the region of 1600–1400 cm−1 are due to the presence of the asymmetrical and symmetrical stretching modes of the framework O–C–O in the MIL-101, which are the typical characteristic peaks of MIL-101 MOFs.35 In addition, the apparent peak at 540 cm−1 is related to the Fe–O vibration,36 and the peaks at 3456 cm−1 and 3373 cm−1 were ascribed to the asymmetrical and symmetrical stretching vibration absorption of the amine groups, respectively. Meanwhile, the bands at 1626 cm−1 and 1337 cm−1 are attributed to σ (N–H) and ν (C–N), respectively.37 NH2-MIL-101(Fe) structure has been further demonstrated by using Raman spectrum analysis (Fig. S1 in the ESI†). The band at 1611 cm−1 is due to the C–O stretching, and the 1425 cm−1 is associated with the out-of-phase stretching modes of the carboxylate group. In addition, a new band at 1133 cm−1, is probably due to a deformation mode involving the carboxylate group, coupled with a C–C stretching mode and the C–H stretching region, where only three components are observed at 860, 810 and 630 cm−1.38–40 Therefore, the results of XRD analysis, FTIR, and Raman spectra clearly confirm the formation of NH2-MIL-101(Fe) structure.
To further study the surface components and chemical states of as-prepared NH2-MIL-101(Fe) hexagonal microspindle, the samples are characterized by XPS. From the survey spectrum in Fig. 2a, it is obvious that the sample contains Fe, N, O and C elements, which is well consistent with the EDS results. In Fig. 2b, the deconvolution of the C 1s peak results in three peaks at about 284.8, 286.1, and 288.6 eV, corresponding to C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively.41 Fig. 2c exhibits a single and strong peak at a binding energy of about 531.6 eV, attributed to O 1s. In Fig. 2d, the spectrum of Fe 2p for NH2-MIL-101(Fe) shows two peaks at 711.9 and 725.4 eV are Fe 2p3/2 and Fe 2p1/2, respectively, which indicates the presence of Fe(III), and the satellite peak at the binding energy of 717.2 eV is also corresponding to Fe(III).42 To evaluate the electron spin state of the NH2-MIL-101(Fe) hexagonal microspindle, ESR measurement was carried out, which is a sensitive measurement of the resonant field and a good way of characterizing the local chemical (i.e. electronic) environment of the unpaired electron.43 A strong and broad single ESR signal is observed for the samples (Fig. S2 in the ESI†), indicating that the valence state of iron ion in the crystal is FeIII.44,45 Moreover, during the fabricated process, the state of Fe element did not change.
O groups, respectively.41 Fig. 2c exhibits a single and strong peak at a binding energy of about 531.6 eV, attributed to O 1s. In Fig. 2d, the spectrum of Fe 2p for NH2-MIL-101(Fe) shows two peaks at 711.9 and 725.4 eV are Fe 2p3/2 and Fe 2p1/2, respectively, which indicates the presence of Fe(III), and the satellite peak at the binding energy of 717.2 eV is also corresponding to Fe(III).42 To evaluate the electron spin state of the NH2-MIL-101(Fe) hexagonal microspindle, ESR measurement was carried out, which is a sensitive measurement of the resonant field and a good way of characterizing the local chemical (i.e. electronic) environment of the unpaired electron.43 A strong and broad single ESR signal is observed for the samples (Fig. S2 in the ESI†), indicating that the valence state of iron ion in the crystal is FeIII.44,45 Moreover, during the fabricated process, the state of Fe element did not change.
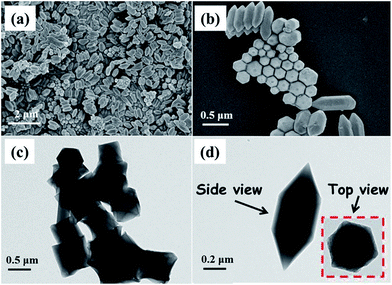
The morphology and particle sizes of the NH2-MIL-101(Fe) sample were observed by FE-SEM. As shown in Fig. 3a, the microspindle NH2-MIL-101(Fe) has been successfully fabricated on a large scale. Fig. 3b shows that the fabricated material consists of hexagonal microspindle crystals with 0.5–1.2 μm of the length, and 100–300 nm of the width. The composition analysis by EDX measurement (Fig. S3 in the ESI†) shows the presence of Fe, C and O elements in the as-prepared sample, and the apparent atomic ratio of C to O is about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is consistent with the stoichiometric composition of organic linkers H2ATA. The morphology of NH2-MIL-101(Fe) was further confirmed by the transmission electron microscopy (TEM) observation. Fig. 2c and d indicate that the sample is composed of hexagonal microspindle with solid structure. From the side view, it can be seen that the outline is regular hexagon, which is consistent with the SEM results.
1, which is consistent with the stoichiometric composition of organic linkers H2ATA. The morphology of NH2-MIL-101(Fe) was further confirmed by the transmission electron microscopy (TEM) observation. Fig. 2c and d indicate that the sample is composed of hexagonal microspindle with solid structure. From the side view, it can be seen that the outline is regular hexagon, which is consistent with the SEM results.
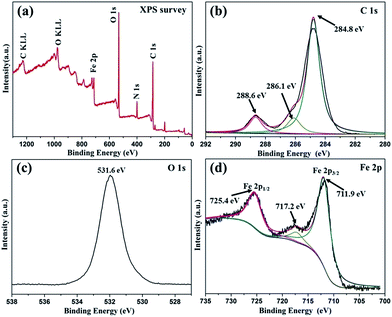 | ||
| Fig. 3 The XPS spectra of the NH2-MIL-101(Fe). (a) Survey of the sample, (b) C 1s spectrum, (c) O 1s spectrum and (d) Fe 2p spectrum. | ||
The optical properties of the NH2-MIL-101(Fe) hexagonal microspindles are characterized by UV-vis absorption spectroscopy. In Fig. 4a, NH2-MIL-101(Fe) displays more broad and intense absorption in the region of visible light than that of MIL-101(Fe). Meanwhile, it could be observed that the color of NH2-MIL-101(Fe) is darker than that of MIL-101(Fe), suggesting that NH2-MIL-101(Fe) possesses better photo-response ability than MIL-101(Fe). The band at around 260–310 nm can be ascribed to electron transfer from the oxygen to iron charge of isolated iron in an octahedral coordination environment, while the other band in the visible-light region can be ascribed to the ligand-based absorption and existence of Fe3O clusters in NH2-MIL-101(Fe).46 The band gap energies of the samples can be calculated from Tauc's plots using (αhν) = A(hν − Eg)n/2, where α is the absorption coefficient near the absorption edge, h is Planck's constant, A is a constant, ν is the light frequency, Eg is the absorption band-gap energy, and n is 1 and 4 for a direct- and indirect-band-gap semiconductor, respectively.47 The Fig. 4b shows the plot of (αhν)1/2 versus hν of the samples, and the equation gives Eg by estimating the intercept of the tangent according to the plot. The band gap of NH2-MIL-101(Fe) hexagonal microspindles is estimated to be about 1.32 eV.
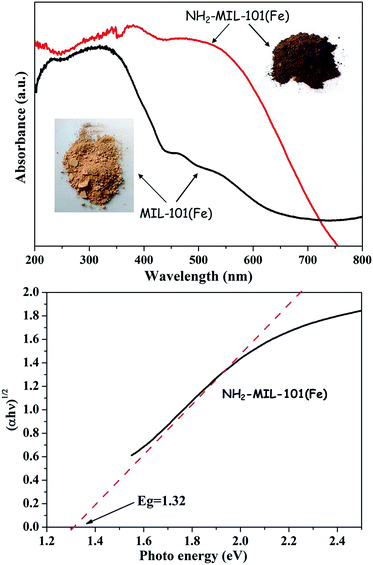 | ||
| Fig. 4 (a) UV spectra of MIL-101(Fe) and NH2-MIL-101(Fe). (b) The main absorption edge of NH2-MIL-101(Fe). | ||
3.2 Photocatalytic activity analysis
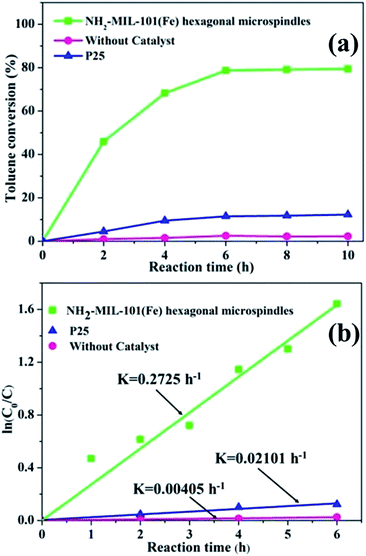
Fig. 5a illustrates the photocatalytic degradation of toluene over different samples under visible light irradiation. For P25 (Degussa), the toluene conversion was about 11% after 10 h reaction time, while for the obtained NH2-MIL-101(Fe) hexagonal microspindles photocatalyst, the toluene conversion could reach about 79.4% after 10 h. The activity of NH2-MIL-101(Fe) catalyst is higher than nano-BiVO4/TiO2 and quantum-BiVO4, but lower than quantum-BiVO4/TiO2 reported recently.48 In Fig. 5b, the kinetic curves of the different samples follow the model of pseudo-first-order reaction. The observed kinetic constants over NH2-MIL-101(Fe) hexagonal microspindles (0.273 h−1) are approximately 13 times as large as P25 (0.021 h−1), which are superior to the previously reported nano-BiVO4/TiO2 (0.203 h−1), quantum-BiVO4 (0.252 h−1), but lower than quantum-BiVO4/TiO2(0.365 h−1).48 The result indicates that the NH2-MIL-101(Fe) hexagonal microspindles exhibited much higher activity than P25 under visible light irradiation. The reasons of enhanced photocatalytic ability include: (1) NH2-MIL-101(Fe) hexagonal microspindles exhibit significantly improved photo-response in the region of visible light; (2) organic linker to metal cluster charge transfer (LCCT) of NH2-MIL-101(Fe) would increase photo-charge separation efficiency.3.3 Mechanism of enhanced photocatalytic activity
In situ FTIR technique could be employed to monitor the surface adsorbed species of catalysts and their transient events occurring on the catalyst during the reaction, which will give an important insight into the reaction mechanism.49 Fig. 6 shows the FTIR spectra collected during the degradation of gaseous toluene over the NH2-MIL-101(Fe) hexagonal microspindles. The peaks of 3079 and 3038 cm−1 can be attributed to the C–H stretching vibration of aromatic ring, while 2937 and 2881 cm−1 could be ascribed to the symmetric and asymmetric C–H stretching vibrations of methyl groups, respectively. In addition, the vibration of aromatic ring is associated with bands at 1609 and 1496 cm−1.50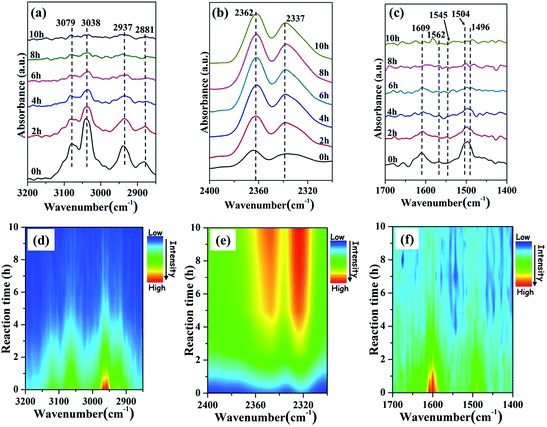 | ||
| Fig. 6 The FTIR spectra of degradation process for gaseous toluene over the NH2-MIL-101(Fe) hexagonal microspindles in the different regions (a–c) and the corresponding time-domain IR spectra (d–f). | ||
Upon irradiation, the characteristic peaks of toluene gradually decreased during the catalytic degradation process. After 10 h reaction, the toluene degradation ratio is as high as 79.4%. Meanwhile, the characteristic peak area corresponding to CO2 (2362 and 2337 cm−1) increased gradually. Furthermore, some intermediate products also formed during the reaction process after 2 h. Generally, the process of toluene oxidation may include the step of forming benzaldehyde as the reaction proceeded.51 However, the weakly adsorbed benzaldehyde could easily be oxidized to benzoic acid by reacting with induced hydroxyl groups on the surface of the catalyst, which would then retain on it firmly, leading to the inactivation of catalyst. The observed new peaks at 1504, 1545 and 1562 cm−1, which are attributed to asymmetric stretching vibration of the carboxylate group COO−, implies the presence of some benzoic acid.48,52,53
On the basis of the above analysis, the mechanism of the photocatalytic degradation for gaseous toluene over the NH2-MIL-101(Fe) hexagonal microspindles can be illustrated in Fig. 7. Generally, there are two excitation pathways in the amine-functionalized metal (M)-containing MOFs:46 (1) the NH2 functionality can absorb visible light and then the photoelectrons would transfer from the organic linker to the M–O clusters, which is denoted as LCCT mechanism; (2) the M–O clusters can directly absorb visible light, leading to photo-generated charge carriers.12 The two pathways can play synergetic roles in exciting these amino-functionalized Fe-based MOFs. When the NH2-MIL-101(Fe) hexagonal microspindles was irradiated by visible light, both of amine-functionalized organic linker and Fe–O clusters could be excited to produce photo-induced electrons and holes. Then the photo-generated electrons produced at amine-clusters transfer to the surface of Fe–O clusters based on LCCT mechanism, meanwhile Fe–O clusters could be excited like semiconductor photocatalyst. After that, the charge carriers transfer to the surface of catalyst, and generate some active species (·OH and ·O2−), which then react with adsorbed toluene, leading to some intermediate products, such as benzoic acid and benzaldehyde. Finally, these species would be mineralized to H2O and CO2.
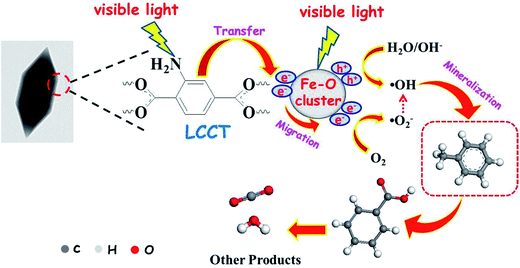 | ||
| Fig. 7 Proposed mechanism for photodegradation of toluene over the NH2-MIL-101(Fe) hexagonal microspindles under visible light irradiation. | ||
4. Conclusions
In summary, the NH2-MIL-101(Fe) hexagonal microspindles were synthesized via the solvothermal method. The results demonstrate that 2-amine-1,4-benzene dicarboxylate (NH2-BDC) anions and inorganic trimmers link together to form basic mesoporous cages unit and then assemble to grow hexagonal microspindles by coordinating interaction. Furthermore, we applied NH2-MIL-101(Fe) in photocatalytic degradation of gaseous toluene under visible light irradiations for the first time. The as-prepared NH2-MIL-101(Fe) hexagonal microspindles exhibit great photocatalytic activity for gaseous toluene under visible light illumination. The conversion efficiency is up to 79.4% under the adopted experimental condition, which is as high as that of the commercial photocatalysts. In addition, the catalytic reaction of gaseous toluene was explored by in situ FTIR, which could clearly display production of the intermediate such as benzaldehyde, benzoic acid, and final mineralized production such as CO2 and H2O during the degradation procedure. These results highlight the great potential of MOFs as photocatalysts for VOCs removal, which may be applied to air purification.Acknowledgements
This work was supported financially by National Nature Science Foundation of China (No. 21377015) and the Key Laboratory of Industrial Ecology and Environmental Engineering, China Ministry of Education.Notes and references
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- D. N. Dybtsev, H. Chun, S. H. Yoon, D. Kim and K. Kim, J. Am. Chem. Soc., 2004, 126, 32–33 CrossRef CAS PubMed.
- O. K. Farha, A. Ö. Yazaydın, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem., 2010, 2, 944–948 CrossRef CAS PubMed.
- A. M. Ebrahim, J. Jagiello and T. J. Bandosz, J. Mater. Chem. A, 2015, 3, 8194–8204 CAS.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 118, 1418–1421 CrossRef.
- L. Hamon, P. L. Llewellyn, T. Devic, A. Ghoufi, G. Clet, V. Guillerm, G. D. Pirngruber, G. Maurin, C. Serre and G. Driver, J. Am. Chem. Soc., 2009, 131, 17490–17499 CrossRef CAS PubMed.
- C.-C. Wang, J.-R. Li, X.-L. Lv, Y.-Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831–2867 CAS.
- L. H. Wee, N. Janssens, S. P. Sree, C. Wiktor, E. Gobechiya, R. A. Fischer, C. E. Kirschhock and J. A. Martens, Nanoscale, 2014, 6, 2056–2060 RSC.
- H. L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351–3370 RSC.
- M. Alvaro, E. Carbonell, B. Ferrer, F. X. Llabres i Xamena and H. Garcia, Chem.–Eur. J., 2007, 13, 5106–5112 CrossRef CAS PubMed.
- F. X. Llabrés i Xamena, A. Corma and H. Garcia, J. Phys. Chem. C, 2007, 111, 80–85 Search PubMed.
- D. Sun, L. Ye and Z. Li, Appl. Catal., B, 2015, 164, 428–432 CrossRef CAS.
- Y. Horiuchi, T. Toyao, M. Saito, K. Mochizuki, M. Iwata, H. Higashimura, M. Anpo and M. Matsuoka, J. Phys. Chem. C, 2012, 116, 20848–20853 CAS.
- Y. Lin, C. Kong and L. Chen, RSC Adv., 2012, 2, 6417–6419 RSC.
- M. C. Das, H. Xu, Z. Wang, G. Srinivas, W. Zhou, Y. F. Yue, V. N. Nesterov, G. Qian and B. Chen, Chem. Commun., 2011, 47, 11715–11717 RSC.
- H. R. Abid, H. M. Ang and S. Wang, Nanoscale, 2012, 4, 3089–3094 RSC.
- W. T. Xu, L. Ma, F. Ke, F. M. Peng, G. S. Xu, Y. H. Shen, J. F. Zhu, L. G. Qiu and Y. P. Yuan, Dalton Trans., 2014, 43, 3792–3798 RSC.
- Y. Zhang, G. Li, H. Lu, Q. Lv and Z. Sun, RSC Adv., 2014, 4, 7594–7600 RSC.
- K. G. Laurier, F. Vermoortele, R. Ameloot, D. E. de Vos, J. Hofkens and M. B. Roeffaers, J. Am. Chem. Soc., 2013, 135, 14488–14491 CrossRef CAS PubMed.
- H.-J. Sedjame, C. Fontaine, G. Lafaye and J. Barbier Jr, Appl. Catal., B, 2014, 144, 233–242 CrossRef CAS.
- L. Malherbe and C. Mandin, Atmos. Environ., 2007, 41, 6322–6330 CrossRef CAS.
- H. M. Liang and C. M. Liao, Chemosphere, 2007, 68, 781–789 CrossRef CAS PubMed.
- Q. Wang, M. Zhang, C. Chen, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7976–7979 CrossRef CAS PubMed.
- K. Parida, N. Sahu, A. Tripathi and V. Kamble, Environ. Sci. Technol., 2010, 44, 4155–4160 CrossRef CAS PubMed.
- B. Liu, X. Li, Q. Zhao, J. Ke, J. Liu, S. Liu and M. Tade, J. Colloid Interface Sci., 2015, 438, 1–6 CrossRef CAS PubMed.
- W. Hou and S. B. Cronin, Adv. Funct. Mater., 2013, 23, 1612–1619 CrossRef CAS.
- L. Rizzo, D. Sannino, V. Vaiano, O. Sacco, A. Scarpa and D. Pietrogiacomi, Appl. Catal., B, 2014, 144, 369–378 CrossRef CAS.
- B. Qiu, M. Xing and J. Zhang, J. Am. Chem. Soc., 2014, 136, 5852–5855 CrossRef CAS PubMed.
- X. An, J. C. Yu, Y. Wang, Y. Hu, X. Yu and G. Zhang, J. Mater. Chem., 2012, 22, 8525–8531 RSC.
- D. Wang and Z. Li, Catal. Sci. Technol., 2015, 5, 1623–1628 CAS.
- S. Bauer, C. Serre, T. Devic, P. Horcajada, J. Marrot, G. Férey and N. Stock, Inorg. Chem., 2008, 47, 7568–7576 CrossRef CAS PubMed.
- Y. Shen, Q. Zhao, X. Li, D. Yuan, Y. Hou and S. Liu, J. Hazard. Mater., 2012, 241–242, 472–477 CrossRef CAS PubMed.
- J. Sun, G. Yu, Q. Huo, Q. Kan and J. Guan, RSC Adv., 2014, 4, 38048–38054 RSC.
- E. V. Ramos-Fernandez, C. Pieters, B. van der Linden, J. Juan-Alcañiz, P. Serra-Crespo, M. W. G. M. Verhoeven, H. Niemantsverdriet, J. Gascon and F. Kapteijn, J. Catal., 2012, 289, 42–52 CrossRef CAS.
- T. A. Vu, G. H. Le, C. D. Dao, L. Q. Dang, K. T. Nguyen, P. T. Dang, H. T. Tran, Q. T. Duong, T. V. Nguyen and G. D. Lee, RSC Adv., 2014, 4, 41185–41194 RSC.
- A. Modrow, D. Zargarani, R. Herges and N. Stock, Dalton Trans., 2012, 41, 8690–8696 RSC.
- L. Shen, S. Liang, W. Wu, R. Liang and L. Wu, Dalton Trans., 2013, 42, 13649–13657 RSC.
- O. V. Zalomaeva, K. A. Kovalenko, Y. A. Chesalov, M. S. Mel'gunov, V. I. Zaikovskii, V. V. Kaichev, A. B. Sorokin, O. A. Kholdeeva and V. P. Fedin, Dalton Trans., 2011, 40, 1441–1444 RSC.
- S. S. Balula, C. M. Granadeiro, A. D. S. Barbosa, I. C. M. S. Santos and L. Cunha-Silva, Catal. Today, 2013, 210, 142–148 CrossRef CAS.
- S. Bordiga, C. Lamberti, G. Ricchiardi, L. Regli, F. Bonino, A. Damin, K.-P. Lillerud, M. Bjorgen and A. Zecchina, Chem. Commun., 2004, 2300–2301 RSC.
- Y. Wu, H. Luo and H. Wang, RSC Adv., 2014, 4, 40435–40438 RSC.
- H. Wang, F. Yin, G. Li, B. Chen and Z. Wang, Int. J. Hydrogen Energy, 2014, 39, 16179–16186 CrossRef CAS.
- S. Guo, X. Li, H. Wang, F. Dong and Z. Wu, J. Colloid Interface Sci., 2012, 369, 373–380 CrossRef CAS PubMed.
- M. Nishikawa, Y. Mitani and Y. Nosaka, J. Phys. Chem. C, 2012, 116, 14900–14907 CAS.
- S. Hernández-Anzaldo, N. Sánchez-Morales, R. Zamorano-Ulloa, R. Escudero, M. de Jesús Rosales Hoz and Y. Reyes-Ortega, J. Mol. Struct., 2013, 1040, 39–46 CrossRef.
- D. Wang, R. Huang, W. Liu, D. Sun and Z. Li, ACS Catal., 2014, 4, 4254–4260 CrossRef CAS.
- S. Li, Y.-H. Lin, B.-P. Zhang, Y. Wang and C.-W. Nan, J. Phys. Chem. C, 2010, 114, 2903–2908 CAS.
- J. Sun, X. Li, Q. Zhao, M. O. Tadé and S. Liu, J. Mater. Chem. A, 2015, 3, 21655–21663 CAS.
- X. Li, X. Zou, Z. Qu, Q. Zhao and L. Wang, Chemosphere, 2011, 83, 674–679 CrossRef CAS PubMed.
- X. Li, Z. Zhu, Q. Zhao and L. Wang, J. Hazard. Mater., 2011, 186, 2089–2096 CrossRef CAS PubMed.
- Q. Yan, X. Li, Q. Zhao and G. Chen, J. Hazard. Mater., 2012, 209–210, 385–391 CrossRef CAS PubMed.
- A. J. Maira, J. M. Coronado, V. Augugliaro, K. L. Yeung, J. C. Conesa and J. Soria, J. Catal., 2001, 202, 413–420 CrossRef CAS.
- M. D. Hernández-Alonso, I. Tejedor-Tejedor, J. M. Coronado and M. A. Anderson, Appl. Catal., B, 2011, 101, 283–293 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23154j |
| This journal is © The Royal Society of Chemistry 2016 |