Preparation and characterization of single crystalline anatase TiO2 films on LSAT (001) by MOCVD
Abstract
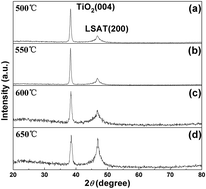
TiO2 thin films with anatase structure have been prepared on [LaAlO3]0.3[SrAl0.5Ta0.5O3]0.7 (LSAT) (001) substrates by metalorganic chemical vapor deposition (MOCVD) in the substrate temperature range of 500–650 °C. Tetrakis-dimethylamino titanium (TDMAT) is used as the organometallic source and oxygen as oxidant. Structural and optical properties of the films as well as the epitaxial mechanism have been investigated by X-ray diffraction (XRD), transmission electron microscopy (TEM) and optical transmittance spectra. The measurements and analyses show that the TiO2 film grown at 550 °C exhibits the best crystallinity with anatase structure. The obtained TiO2 film is a single-crystalline epitaxial film with no twins and very few defects. The heteroepitaxial relationship is determined as TiO2 (001) ‖ LSAT (001) with TiO2 [100] ‖ LSAT 〈100〉. The average transmittance of the film deposited at 550 °C in the visible wavelength is about 84% with an optical band gap of about 3.27 eV.

 Please wait while we load your content...
Please wait while we load your content...