BODIPY based self-healing fluorescent gel formation via acylhydrazone linkage†
Abstract
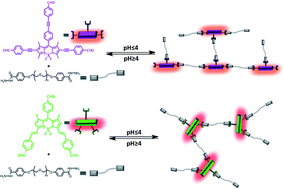
Polymeric BODIPY derivatives with reactive functional groups undergo reversible formation of covalent bonds leading to self-healing properties. Cross-reactivity of acyl-hydrazine and aldehyde moieties forms the basis of dynamic covalent chemistry leading to sol–gel transition in this series of compounds. In addition, thin layers of absorbers show efficient energy transfer indicating a potential in solar concentration.

 Please wait while we load your content...
Please wait while we load your content...