99mTc-labeled and gadolinium-chelated transferrin enhances the sensitivity and specificity of dual-modality SPECT/MR imaging of breast cancer
Abstract
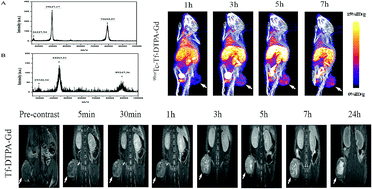
A tumor-targeting dual-modal probe, 99mTc-labeled and gadolinium (Gd)-chelated transferrin was explored to improve the imaging of solid tumors such as breast cancer. In the present study, the Gd was successfully chelated to transferrin with 26 Gd loaded per protein and calculated longitudinal relaxivity r1 4.34 mM−1 s−1 per Gd, while that of Gd–DTPA was 4.19 mM−1 s−1. 99mTc was labeled to Tf–DTPA–Gd, yielding a labeling rate of approximately 96% and a radiochemical purity of greater than 96%. For the binding between 99mTc–Tf–DTPA–Gd and the transferrin receptor on the surface of 4T1 cells, the equilibrium dissociation constant (Kd) was 3120 ± 600.60 nmol L−1, and the half-inhibition concentration (IC50) was 23.46 ± 1.36 nmol L−1. Furthermore, Tf–DTPA–Gd improved the diagnostic efficiency of breast cancer with the relatively enhanced signal (195.25%) in T1-weighted MR imaging, compared to 131.75% for Magnevist. SPECT/CT imaging with 99mTc–Tf–DTPA–Gd indicated that the tumor imaging was clearest 5 h after the injection. The mean calculated half-lives of 99mTc–Tf–DTPA–Gd in blood were 6.12 and 69.32 minutes, respectively. The high uptake of radioactivity in liver and kidneys suggested that 99mTc–Tf–DTPA–Gd was predominantly metabolized and cleared by the liver and kidneys. This dual-modal probe can be effectively utilized for the specific imaging of breast tumor via SPECT and MR.

 Please wait while we load your content...
Please wait while we load your content...