Greener synthesis and characterization, antimicrobial and cytotoxicity studies of gold nanoparticles of novel shapes and sizes†
Abstract
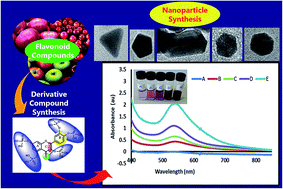
We hereby present a novel approach for the synthesis of gold nanoparticles (AuNPs) using water soluble, naturally-derived flavonoids. Quercetin pentaphosphate (QPP), quercetin sulfonic acid (QSA) and apigenin triphosphate (ATRP) were utilized as reducing agents and stabilizers for the gold nanoparticle synthesis. Synthesis was achieved at room temperature using water as a solvent and it requires no capping agents. The AuNPs were characterized using UV-vis, X-ray diffraction (XRD), transmission electron microscopy (TEM), energy dispersive absorption spectroscopy (EDS), high resolution transmission electron microscopy (HR-TEM) and selected area electron diffraction (SAED). The resulting AuNPs were spherical, triangular, cubicle, hexagonal and rectangular in shape. The average particle sizes of 4.85 nm, 9.56 nm and 13.54 nm were obtained for the nanoparticles derived from QPP, ATRP and QSA respectively. The surface plasmon resonance peak of the AuNPs derived from QSA, ATRP and QPP was observed at 541 nm, 544 nm and 547 nm respectively. The AuNPs exhibited excellent antibacterial activities of 99.9%, 100% and 99.9% growth inhibition for Escherichia coli ATCC® 25922™, Staphylococcus epidermidis ATCC® 12228™ and Citrobacter freundii ATCC® 8090 at 104 cfu inoculations. The AuNPs were observed to retain stability after 150 days compared to those reported using conventional approaches of 30 days. This work also provides insights into the mechanism of flavonoid-based nanoparticle synthesis while eliminating the use of hazardous and toxic organic solvents and adopting the use of water as a solvent.

 Please wait while we load your content...
Please wait while we load your content...