Effect of RF power on the structural, optical and gas sensing properties of RF-sputtered Al doped ZnO thin films
Abstract
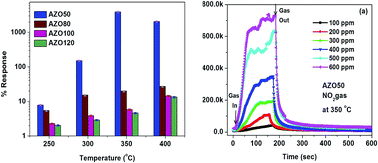
The effect of Radio Frequency (RF) power on the properties of magnetron sputtered Al doped ZnO thin films and the related sensor properties are investigated. A series of 2 wt% Al doped ZnO; Zn0.98Al0.02O (AZO) thin films prepared with magnetron sputtering at different RF powers, are examined. The structural results reveal a good adhesive nature of thin films with quartz substrates as well as increasing thickness of the films with increasing RF power. Besides, the increasing RF power is found to improve the crystallinity and grain growth as confirmed by X-ray diffraction. On the other hand, the optical transmittance is significantly influenced by the RF power, where the transparency values achieved are higher than 82% for all the AZO thin films and the estimated optical band gap energy is found to decrease with RF power due to an increase in the crystallite size as well as the film thickness. In addition, the defect induced luminescence at low temperature (77 K) and room temperature (300 K) was studied through photoluminescence spectroscopy, it is found that the defect density of electronic states of the Al3+ ion increases with an increase of RF power due to the increase in the thickness of the film and the crystallite size. The gas sensing behavior of AZO films was studied for NO2 at 350 °C. The AZO film shows a good response towards NO2 gas and also a good relationship between the response and the NO2 concentration, which is modeled using an empirical formula. The sensing mechanism of NO2 is discussed.

 Please wait while we load your content...
Please wait while we load your content...