Synthesis and characterization of biodegradable copolymer derived from dextrin and poly(vinyl acetate) via atom transfer radical polymerization†
Abstract
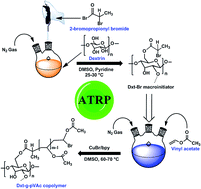
This article reports the development of a dextrin-based amphiphilic biodegradable graft copolymer (Dxt-g-pVAc) via atom transfer radical polymerization (ATRP). The copolymer was synthesized by a “grafting from” approach using 2-bromopropionyl bromide as a bromo compound, vinyl acetate as a monomer and CuBr/bpy as an activator. The monomer concentration, temperature and time were varied to obtain the copolymer (Dxt-g-pVAc) with a relatively high molecular weight (Mn) and low polydispersity index (PDI). The synthesized copolymer was well characterized using FTIR and 1H NMR spectral analyses, GPC, TGA, AFM, FESEM and EDAX analyses. The degree of substitution (DS) of dextrin was determined using 1H NMR spectroscopy. A GPC kinetics study confirmed the livingness of the ATRP of vinyl acetate on dextrin. Scanning electron microscopy (SEM) analysis showed that the copolymer has a microporous morphology, whereas an atomic force microscopy (AFM) study indicated that the roughness of the copolymer decreased after the copolymerization of poly(vinyl acetate). The gel characteristics of the copolymer were investigated using rheological parameters. A biodegradation study using hen egg lysozyme confirmed the biodegradability of the copolymer.

 Please wait while we load your content...
Please wait while we load your content...