Mechanistic insights into the activation process in electrocatalytic ethanol oxidation by phosphomolybdic acid-stabilised palladium(0) nanoparticles (PdNPs@PMo12)
Abstract
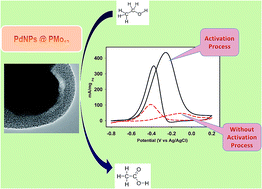
A Keggin type polyoxometalate (POM), phosphomolybdic acid (PMo12), has been employed to encapsulate and stabilise pseudo-spherical Pd(0) nanoparticles (PdNPs). The resulting nanohybrid phosphomolybdic acid-stabilised PdNPs (PdNPs@PMo12) were characterised by transmission electron microscopy (TEM), high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM), energy-dispersive X-ray spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR) and powder X-ray diffraction (XRD). The PdNPs@PMo12 were used as an electrocatalyst for ethanol oxidation in alkaline media and the best electrocatalytic activity was assessed by applying an activation process. The electrochemical data (chronoamperometry, cyclic voltammetry and linear sweep voltammetry) allowed us to attribute the superior performance of the nanohybrid to the generation of Pd–OHads sites at the surface of the PdNPs@PMo12, reinforced by the formation of a new molybdenum species on the surface of the electrocatalyst.

 Please wait while we load your content...
Please wait while we load your content...