The excellent cycling stability and superior rate capability of polypyrrole as the anode material for rechargeable sodium ion batteries
Abstract
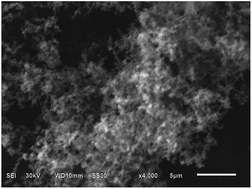
Polypyrrole with a submicrostructure has been successfully synthesized via a simple chemical oxidative polymerization. As characterized by X-ray diffraction, Fourier transform infrared (FTIR) spectroscopy, and scanning electron microscopy (SEM), the submicron polypyrrole shows a fluffy structure comprised chains composed of submicron particles. The submicron polypyrrole shows excellent electrochemical performance as the anode material for sodium ion batteries, which is much better than bulk polypyrrole. It displays a stable discharge capacity of 183 mA h g−1 at 400 mA g−1 after 100 cycles, whereas only 34.8 mA h g−1 for bulk polypyrrole under the same conditions. Furthermore, the submicron polypyrrole shows an extremely stable storage capacity during long term cycling even at super high rates. Except the irreversible capacity for the first cycle, the specific capacity remains stable and still maintains at 84 mA h g−1 after 500 cycles at 14 400 mA g−1. The remarkable electrochemical performance greatly suggests that the submicron polypyrrole is an advanced anode material for sodium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...