Phosphonium based ionic liquids-stabilizing or destabilizing agents for collagen?†
Aafiya Tarannum,
Charuvaka Muvva,
Ami Mehta,
J. Raghava Rao and
N. Nishad Fathima*
Chemical Laboratory, CSIR-Central Leather Research Institute, Adyar, Chennai 600020, India. E-mail: nishad@clri.res.in; nishad.naveed@gmail.com; Fax: +91 44 24911589; Tel: +91 44 24411630
First published on 15th December 2015
Abstract
Collagen aids in the preparation of biomaterials and its stability is a key factor for these applications. In this study, phosphonium based ionic liquids (PILs) were explored for their stability effects on collagen. The rheology of collagen and UV and fluorescence spectra slackens up with the increasing concentrations. The CD and FT-IR spectra unveil the interaction of the ions in the ionic liquid with the collagen exhibiting denaturation at higher levels. At the inter-fibrillar level, concentration dependent distortion in the banding pattern of RTT collagen fiber was observed due to the chaotropicity of ions. Molecular modeling studies also reveal the ions effect on the stability of collagen and the structural deformation at higher concentrations. Our results show that the ions play an active role on the level of interaction with protein towards the stabilization or destabilization of collagen.
1. Introduction
Ionic liquids (ILs) are molten salts at room temperature. They are purely ionic, consisting solely of cations and anions.1 A wide range of ionic liquids, viz., ammonium, phosphonium, choline, imidazolium, pyrrolidinium, pyridinium and sulfonium,2 have been deliberately employed as the electrolyte phase for biosensors,3 medium for green synthesis4 and protein preservation.5 They have demonstrated the stabilization of proteins by varying the cations, anions and alkyl chains.6Phosphonium cations paired with chloride and sulfonyl imide anions exhibit strong ion association, low viscosity and volatility.7 They have shown improved thermal stability and superior properties when compared to their ammonium based counter parts.8 When phosphonium, ammonium and imidazolium are taken into consideration, the phosphonium RTILs prominently have lower surface tension and varying the anions has a negligible effect.9
Changes in the native structure of BSA,10 myoglobin11 and insulin12 with different ionic liquids have been explored. ILs serves as a strong stabilizing medium for cytochrome C,13 lysozyme14 and nucleic acids15 offering extended storage periods without loss of activity. It was reported that choline ILs have been used considerably for protein-based pharmaceutical preparations and cellular therapies.16 The anion of the IL has an impact on the stability and activity of the enzyme causing conformational changes as they strongly interact to alter the enzymes functionality.17
Collagen has extensive applications for the preparation of biomaterials. The extended use of collagen brings out the need to understand the mechanism of stabilization because they have gained significance in industrial and biological applications.
Interestingly, many ILs have been studied with collagen at different hierarchical ordering. It has been reported that choline DHP, a stabilizing agent for collagen, can be used as a potential crosslinker,18 whereas the ammonium and imidazolium19 ILs have been denoted as destabilizing agents.
The purpose of the current investigation is to explore the stability of collagen with phosphonium based ionic liquids (PILs) and study the effect of PILs on the structure and conformational dynamics of collagen with varying anions, such as tributyl methyl phosphonium methyl sulfate (PMS) and tributyl ethyl phosphonium diethyl phosphate (PEP), using different characterization techniques, viz., viscosity analysis, circular dichroism (CD) studies, Fourier transform infrared (FT-IR) spectroscopy, UV and fluorescence, dimensional stability and impedance measurements. Furthermore, to complement the experimental studies, molecular dynamics simulation studies were performed.
2. Experimental methods
2.1 Materials
Rat tail tendon (RTT) excised from six month old albino rats (Wistar strain) were teased out. Tributyl methyl phosphonium methyl sulphate (PMS) and tributyl ethyl phosphonium diethyl phosphate (PEP) (of 98% purity) were purchased from Ionic Liquid Technologies GmbH (IoLiTec) and used without further purification for this study. Millipore water was used for further studies. All other chemicals were of analytical grade.2.2 Isolation of type I soluble collagen
The tails of six month old albino rats (Wistar strain) were excised as they are a rich source of high purity collagen. The teased collagen fibre was washed with 0.9% NaCl at 4 °C. Collagen was extracted using the acetic acid extraction method20 and precipitated with 5% sodium chloride. The precipitate was collected by centrifugation was dialyzed extensively against 50 mM phosphate buffer. The concentration of collagen in the solution was determined from the hydroxyproline content according to the Woessner method.21 The molar concentration of collagen was determined considering the average molecular weight of collagen as 300 kDa. The extracted collagen was used throughout the studies.2.3 Preparation of collagen treated PILs
The working concentration of collagen was estimated as 0.8 μM at pH 4. Phosphonium ionic liquid, viz., phosphonium methyl sulfate (PMS) and phosphonium diethyl phosphate (PEP) was prepared at different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05, 1
0.05, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 1
5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with collagen in an acetate buffer under stirring for 3 h at 4 °C. The concentration of PILs was varied maintaining the concentration of collagen constant.
10) with collagen in an acetate buffer under stirring for 3 h at 4 °C. The concentration of PILs was varied maintaining the concentration of collagen constant.
2.4 Viscosity measurements
The viscosity of PILs treated collagen was performed using an Ostwald type viscometer with 3 mL capacity. The flow times for the collagen samples were measured after a thermal equilibrium time of 30 min. The collagen concentration was fixed and measurements were carried out with different concentration of ILs. This is based on the flow rate of the collagen solution through the capillary of an Ostwald viscometer. The flow time was measured with a digital stopwatch, each sample was studied in triplicate and the mean was taken. The viscosity (ηrel) was calculated from the equation,| ηrel = η/η0 |
The collagen treated PILs were plotted on the x-axis and ηrel on the y-axis (ηrel = 1/R), (R = additive/collagen).
2.5 UV-visible absorbance studies
PILs treated collagen was analyzed using a UV-1800 Shimadzu (UV-Visible spectrophotometer) with a quartz cuvette of 1.0 cm path length, assuring the proper determination of the baseline. The samples were incubated overnight prior to studying the interactions between native and PILs treated collagen.2.6 Fluorescence measurements
Fluorescence spectra for PILs treated collagen were obtained on a Cary Eclipse Fluorescence Spectrophotometer (Varian, Walnut Creek, USA) in a quartz cell. The excitation wavelength for collagen treated ILs was set at 275 nm and the emission was obtained from 285 to 400 nm with 5 nm slit widths in both the excitation and emission path at a scan rate of 100 nm min−1.2.7 CD spectroscopic studies
Far ultraviolet (190 nm to 260 nm) CD spectra for PILs treated collagen were obtained using a Jasco 815 Circular dichroism spectropolarimeter in a quartz cell of volume 400 μL with a light path of 1 mm and scanned with 0.2 nm intervals at 25 °C under a nitrogen atmosphere with computer-averaged trace of three scans for each samples. The results were plotted with molar-residue-weight ellipticity (deg cm2 dmol−1) versus wavelength in nanometers (nm).2.8 FT-IR studies
Samples for FT-IR analysis were lyophilized under a pressure of 6.4 Pa at −40 °C. Prior to lyophilisation, all the PILs treated collagen samples were frozen by dipping in liquid N2 and kept in the deep freezer for overnight. The wavelength dependent transmission intensity was characterized to observe the interactions between collagen and the ionic liquids using a Jasco FT/IR-4200 (Fourier Transform Infrared Spectrometer) with 60 scans in the range of 4000–400 cm−1 at 25 °C with a resolution of 4 cm−1.2.9 The dimensional stability of RTT collagen fibres with PILs
Rat tail tendons (RTT) treated with ionic liquid for 24 h were observed under a Aven Inc., Digital Mighty Scope, 1.3M (product code: 48708-25, made in Taiwan) of 10× resolution. The changes in the dimensional stability should be monitored.2.10 Dielectric measurements
Impedance analysis for native and PILs treated collagen were carried out to determine the effect of the ionic liquids on the resultant dipole of the protein molecule responding to an alternating electric field by means of a CH Instrumental (USA) electrochemical analyzerCH-model 660 B with a classical three electrode system, wherein a glassy carbon electrode serves as the working electrode, a platinum electrode as the counter electrode and a saturated calomel electrode as the reference electrode. The operating conditions of the dielectric measurements were Init(E) = 90 mV, high frequency (f) = 105 Hz, low frequency (f) = 0.01 Hz, amplitude (V) = 0.005, quiet time = 2 s, cycles (0.01–0.1 Hz) = 1, and cycles (0.001–0.01 Hz) = 1.All the measurements were carried out in triplicate and the average values reported. Dielectric data can be represented in terms of admittance. The admittance Y′′ (unit: Ω−1) can be written as
| Y* = Y′ + jY′′ |
2.11 Molecular modelling studies
To understand the structural behaviour of collagen at different concentrations of PMS and PEP, classical molecular dynamics simulations were performed. The structure of collagen like peptides was constructed using the gen collagen package.22 The molecular dynamics simulation was carried out using the GROMACS (version 4.6.2)23,24 package employing the AMBER03 force field25 for collagen. The geometries of the AMS compound were fully optimized using the B3LYP/6-31G(d,p) level of theory and their electrostatic potentials obtained using same level of theory, using the GAUSSIAN 09 package.26 The force field was generated using the Antechamber module of amber package27,28 and RESP charges were generated for PMS and PEP. Each model was solvated with TIP3P water molecules in a cubic box.29 All models were subjected to energy minimization using the steepest decent method to relax the entire system. The production run was carried out for all the systems for 100 nanoseconds (ns) using 2 femtoseconds time step for integration of equation of motion in NPT ensemble at 300 K and at 1 atmospheric pressure, which was controlled using a V-rescale thermostat and Parrinello–Rahman Barostat, respectively.30–32 The particle mesh Ewald (PME) was used to calculate the electrostatic interaction with a cutoff of 10 Å. The trajectories obtained from the MD simulations were visualized with the help of the VMD package.33 Further analysis of the trajectories was performed with the tools available in the GROMACS package.The peptide sequence for collagen used for modelling in this study is,
GPOGKOGPOGPOGPOGROGPOGAOGHOGSO
GPOGKOGPOGPOGPOGROGPOGAOGHOGSO
GPOGKOGPOGPOGPOGROGPOGAOGHOGSO
This proposed sequence was developed by Object Technology Framework (OTF) using the GENCOLLAGEN package.34 This sequence was chosen for modelling, as these amino acid residues play a significant role in interacting with the additives and it alters the microenvironment of collagen.
3. Results and discussion
3.1 The effect of PILs on the rheology of collagen – viscosity measurements
To understand the influence of PILs on the rheological behaviour of collagen, viscosity measurements were carried out.35 The rheological properties reflect the strength of the inter- and intra-molecular forces; the stronger the force the higher the viscosity will be. The relative viscosity of collagen was studied by varying the concentration of PILs. Fig. S1† exhibits the rheological behaviour for collagen-PILs, which demonstrated the change in the relative viscosity (ηrelative) with the addition of PIL. The ηrelative value was found to increase with increasing PIL concentration. Therefore, the rheology of PEP and PMS treated collagen shows the highest tendency to aggregate. The increasing viscosity with increase in concentration of PILs generally attributes to aggregation.3.2 The effect of PILs on the microenvironment of collagen – UV absorption studies
The sensitivity of tyrosine to its chemical environment makes this amino acid a highly important spectroscopic probe for conformations and dynamics. In this context, the absorption spectra of biomacromolecule have been made the subject of considerable study. In the UV region, the peak at 205 nm indicates the peptide absorption peak in protein, whereas a second weak absorption peak at about 278 nm is due to aromatic amino acids.36As observed from Fig. S2a,† at 278 nm, an increase in the absorbance intensity for phosphonium methyl sulfate (PMS) was observed for C-PMS-1 and C-PMS-2 when compared to native collagen, which might be due to the least interaction of collagen with PMS, whereas in C-PMS-3 and C-PMS-4 the decrease in absorbance intensity indicated changes in the microenvironment of collagen. This might be due to the ions, which interact strongly with collagen at higher concentrations of PMS causing structural deformation.
In addition, Fig. S2b† demonstrates a decrease in the absorbance with an increase in the concentration of C-PEP-2, C-PEP-3 and C-PEP-4, implying the complete loss of the tyrosine environment, which may be due to the direct interaction of PEP, thereby resulting in denaturation of collagen.
3.3 The effect of PILs on the fluorescence spectra of collagen
The tyrosine environment of native collagen and collagen in PILs was analyzed using fluorescence spectroscopy. In Fig. S3a,† phosphonium methyl sulfate (PMS) shows the emission of the tyrosine residues at 305 nm with increased fluorescence up to C-PMS-2. The lack of a characteristic peak for C-PMS-3 and C-PMS-4 records the absence of the tyrosine emission. This could be due to denaturation of collagen at higher concentrations. In Fig. S3b,† phosphonium diethyl phosphate (PEP) shows a strong emission for the tyrosine residue at 305 nm, revealing an increase in intensity till C-PEP-3. The absence of a characteristic peak for C-PEP-4 at 305 nm was observed, which might be due to the movement of tyrosine residues, resulting in defragmentation of molecules tapering the aggregation of collagen.3.4 The effect of PILs on the secondary structure of collagen – CD spectral studies
To understand whether PILs influence the secondary structure of collagen, CD spectral studies on collagen/PILs were carried out.37,38 Fig. 1a and b describes the CD spectra of collagen with two PILs at different concentrations. Phosphonium methyl sulphate (PMS) and phosphonium diethyl phosphate (PEP) alone do not show any spectra in the range of 190–260 nm, indicating that the observed CD spectrum is due to collagen. In the far UV region, pure collagen exhibits its minimum at 197 nm and maximum at 222 nm, authenticating a typical polyproline type II (PPII) conformation. C-PMS-1 and C-PMS-2 recorded an increased ellipticity compared to C-PEP-1 and C-PEP-2 due to the least interactions and lesser hydrogen bonding of collagen with the ions present in PILs. The acerbic change with increasing concentration of PILs, C-PMS/PEP-3 and C-PMS/PEP-4 was shown to decrease in their molar ellipticity values when compared to native collagen, which might be due to the increased hydrogen bonding ability of the ions indicating a strong interaction resulting in the denaturation of the collagen structure. A marginal increase in the molar ellipticity value suggests a negligible effect on the secondary structure of collagen was witnessed for the imidazolium ILs.19 A remarkable increase in the molar ellipticity of collagen treated with choline ILs18 was observed, which indicated the stability of collagen. The different changes in the CD spectra of the collagen treated PILs shows that the interaction with collagen affects the structure of collagen at higher levels.3.5 The effect of PILs on the alteration of functional groups of collagen – FT-IR spectral studies
Infrared (IR) spectroscopy detects the vibrational characteristics of the functional groups in a sample. A functional group tends to absorb IR radiation in a specific wave number (cm−1) range.39,40 The presence or absence of peaks within the region gives structural information regarding the molecule. Collagen shows a characteristic FT-IR spectrum, with absorption bands for N–H and O–H stretching at 3300 cm−1 for amide A, C–H stretching at 2948 cm−1 for amide B, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at 1640 cm−1 for amide I, N–H bending and C–H stretching at 1560 cm−1 for amide II, and carboxyl OH at 1240 cm−1 for amide III. It is known that the amide I and II bands are attributed to the polyproline type II structure of collagen. Fig. 2a and b show the slight shift in the amide A band and O–H stretching to higher frequencies (3280 cm−1) for C-PMS/PEP (1–4), this may have arisen from the least interaction of hydrogen bonds within collagen molecules resulting from the interaction between collagen and PMS/PEP compared to that of native collagen (3279 cm−1). Moreover, the amide I is centred around 1635 cm−1, indicating C
O stretching at 1640 cm−1 for amide I, N–H bending and C–H stretching at 1560 cm−1 for amide II, and carboxyl OH at 1240 cm−1 for amide III. It is known that the amide I and II bands are attributed to the polyproline type II structure of collagen. Fig. 2a and b show the slight shift in the amide A band and O–H stretching to higher frequencies (3280 cm−1) for C-PMS/PEP (1–4), this may have arisen from the least interaction of hydrogen bonds within collagen molecules resulting from the interaction between collagen and PMS/PEP compared to that of native collagen (3279 cm−1). Moreover, the amide I is centred around 1635 cm−1, indicating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching for collagen treated PMS, whereas the shift in the peaks was observed around 1631 cm−1 for C-PEP-3 and C-PEP-4. This might be due to the burying of hydrophilic groups in the interior core of collagen at higher concentrations. N–H bending and C–H stretching for amide II was observed at 1561 cm−1 for native collagen, but there was a variation in the corresponding concentration of C-PMS and C-PEP, which was signalled around 1550 and 1545 cm−1, respectively. However there was an abrupt change witnessed in the amide III band for C-PEP-4 with a shift from 1241 cm−1 to 1224 cm−1 and for C-PMS-4 there was drastic shift witnessed from 1241 cm−1 to 1229 cm−1 indicating a plausible interaction of collagen with the ionic liquids, altering the microenvironment of collagen, which in turn causes disruption in their structures. In the case of imidazolium ILs,19 there was slight shift in the amide I, II and III bands indicating tenuous change in the secondary structure of collagen, whereas the shifts in the choline ILs18 indicated increased physical crosslinks.
O stretching for collagen treated PMS, whereas the shift in the peaks was observed around 1631 cm−1 for C-PEP-3 and C-PEP-4. This might be due to the burying of hydrophilic groups in the interior core of collagen at higher concentrations. N–H bending and C–H stretching for amide II was observed at 1561 cm−1 for native collagen, but there was a variation in the corresponding concentration of C-PMS and C-PEP, which was signalled around 1550 and 1545 cm−1, respectively. However there was an abrupt change witnessed in the amide III band for C-PEP-4 with a shift from 1241 cm−1 to 1224 cm−1 and for C-PMS-4 there was drastic shift witnessed from 1241 cm−1 to 1229 cm−1 indicating a plausible interaction of collagen with the ionic liquids, altering the microenvironment of collagen, which in turn causes disruption in their structures. In the case of imidazolium ILs,19 there was slight shift in the amide I, II and III bands indicating tenuous change in the secondary structure of collagen, whereas the shifts in the choline ILs18 indicated increased physical crosslinks.
The amide bands in the FT-IR spectra are sensitive to the secondary structure of collagen. Changes in the position of the amide of collagen clarifies that the secondary structure of collagen is altered, which indicates aggregation or compactness. From the experimental observations, it was concluded that the ions have a high hydrogen bond forming capability and strongly interact with collagen, which causes the conformational changes in the structure, which can be further confirmed through the molecular dynamics studies.
3.6 The effect of PILs on the dimensional stability of RTT collagen fibres
Rat tail tendons (RTT) are known to be the rich source of type I collagen fibres and were excised from albino rats. It shows the characteristic macroscopic banding pattern in an aqueous medium and reveals the helicity of the fibrils.41 Fig. 3 explicates the treatment of RTT with phosphonium diethyl phosphate (PEP) and phosphonium methyl sulfate (PMS) with varying concentrations (0.05%, 0.5%, 5% and 10%) and unveils the huge impact on the dimensional stability of the RTT collagen fibres. It was observed that there was a remarkable distortion in the wave pattern within the first hour of incubation and no change was observed in the later hours, whereas for imidazolium ILs,19 a slight swelling effect was observed, which may be attributed to the increased surface tension at the water-IL interface. The choline18 ILs shows no swelling effect indicating stabilization. The drastic distortion in wave pattern for PILs could be due to the chaotropicity of ions that alters the structure of the water molecules, leading to agglomeration, defragmentation and destabilization of the RTT collagen fibres.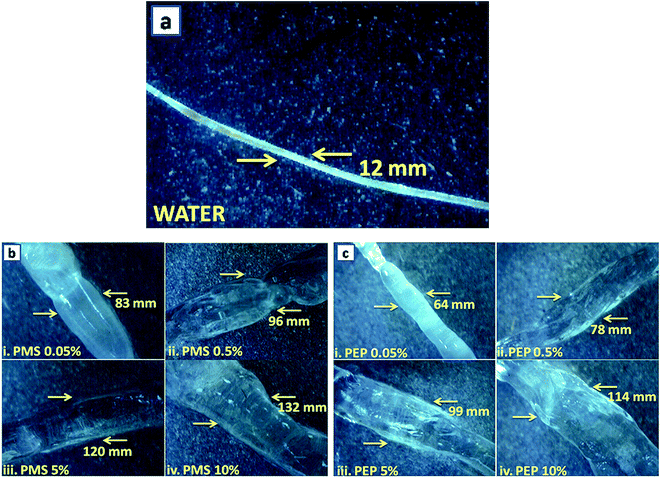 | ||
| Fig. 3 Optical micrographs of (a) water, (b) phosphonium methyl sulfate (PMS) 0.05% to 10% and (c) phosphonium diethyl phosphate (PEP) 0.05% to 10%. | ||
3.7 Dielectric measurements
AC impedance measurements aid in determining the nature of hydrated components in proteins through its dielectric response under an electric field. Collagen composites with different concentrations of PILs were subjected to impedance measurements to determine the dielectric properties and hydration behaviour of biomacromolecules.42,43 The dielectric technique is particularly sensitive for analysing the dynamic characteristics of water. This phenomenon will have an effect on the conformation of collagen in the presence of the phosphonium class of ionic liquids. The Nyquist admittance plot demonstrates the capability to trap electric charge through the steepness of the circles, whereas the Debye relaxation model explains the polarization behaviour as a response to the entrapped electric charge. Fig. 4a shows the Nyquist plot for C-PMS discerning the admittance plotted using real Y′ vs. imaginary Y′′. A concentration dependent increase in permittivity was observed for C-PMS, whereas collagen shows the lowest permittivity. This is attributed to the fact that collagen is a protein with various functional groups, which led to its charged behaviour. For collagen/PMS (1–4), there was an increase in permittivity with an increasing concentration of PMS treated collagen. The Bode plot in Fig. 4b for collagen, C-PMS-1 and C-PMS-2 shows a phase angle at high frequency of about 103 Hz. For C-PMS-3 and C-PMS-4, the phase angle is increased to higher frequencies, from 103 to 105 Hz. This change in frequency owing to the addition of PMS was likely due to interaction of the charged functional groups of collagen with PMS. The result indicates that the dielectric behaviour of collagen-PMS is due to the displacement of charges.Fig. 4c depicts the Nyquist plot for PEP treated collagen, revealing the admittance for biomacromolecule. Native collagen shows the lowest permittivity, whereas the PEP treated collagen shows a concentration dependent permittivity. This may be due to the reorganization of bound water molecules in the proteins and there is strong influence from the additives in it. The Bode plot in Fig. 4d shows a phase angle at high frequency of about 103 Hz for native collagen, C-PEP-1 and C-PEP-2, albeit for C-PEP-3 and C-PEP-4, the phase angle is shifted to higher frequencies from 103 to 105 Hz, suggesting that there is a high probability of charge transfer between the amino acids in the protein, ions present in ionic liquid and water molecules. It clearly explains the bound charges of the protein when comes in contact with the dielectric current resulting in reorientation and reorganization in the network of proteins.
3.8 Molecular dynamics simulation studies
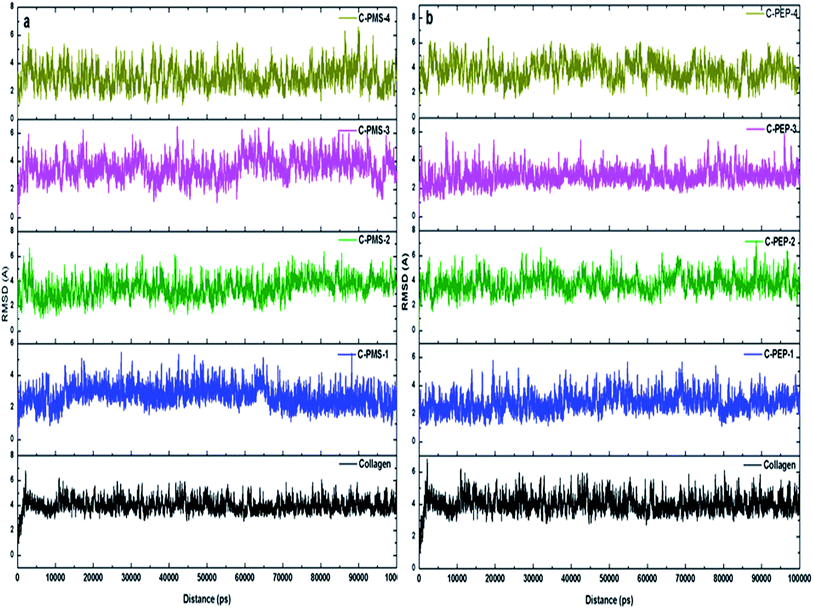
Molecular dynamics simulation studies were carried out for collagen upon its interaction with phosphonium methyl sulphate (PMS) and phosphonium diethyl phosphate (PEP). Molecular docking studies for the choline ILs indicated that the interaction between choline ILs18 and collagen was electrostatic in nature, which is likely to promote stability. In this study, root mean square deviation (RMSD), root mean square fluctuation (RMSF), hydrogen bonding analysis, radial distribution analysis and spatial distribution analysis have been demonstrated to complement the experimental techniques.To understand the effect of PMS and PEP at varied concentrations on the structural stability of collagen, the RMSF of the Cα residues from its time averaged position were computed. Fig. 6a and b illustrates the slight changes in the RMSF of collagen, whereas a huge residual fluctuation was observed for higher concentrations of PMS and PEP, with an increase observed for increasing concentrations of PMS and PEP. These results suggest the noticeable change in the flexibility of collagen upon increasing the concentration of phosphonium ILs.
For C-PMS-1 and C-PMS-2 (anion), only a few hydrogen bonds were observed and there was an increase in the number of hydrogen bonds with an increase in the concentration of PMS, which can be observed from Fig. 7c and d. On average, 20 hydrogen bonds were observed for C-PMS-3 and C-PMS-4. On the other hand for C-PEP-1 to C-PEP-4, there was an increase in the formation of hydrogen bonds upon increasing the concentration of PEP. From the hydrogen bonding analysis, it was evident that at higher concentrations of C-PMS and C-PEP (anion), the number of hydrogen bonds increased drastically leading to strong interactions between collagen and PMS and PEP (anion), which might be the reason for the structural deformation of collagen. It is elucidated that anions play a major role in the structural deformity of collagen, whereas cations show a negligible effect. The anion of the IL has an impact on the protein stability and activity. In general, it was observed that anions having a high hydrogen bond forming capability, strongly interact with protein causing the conformational changes in the structure; the stronger the bonding, the higher the interaction will be with the marked changes in the structure of the proteins.
These outcomes clearly abide with the FT-IR, CD and viscosity analyses, which state that the interaction of collagen with an increased concentration of PEP and PMS leads to collagen denaturation.
Fig. 8c exhibits that the PMS (anion) value of g(r) is high and is within 0.7 nm for C-PMS-1 and C-PMS-2. There was a decrease in the g(r) values with an increase in the concentration of PMS (anion). As observed from Fig. 8d, PEP (anion) witnessed the higher value for a lower concentration of PEP, which was around 0.7 nm. There was a drastic decrease in the g(r) values for C-PEP-3 and C-PEP-4. The results clearly show that the interaction of anions with collagen is more when compared to the cation, as there was acerbic change in the g(r) values for both PMS and PEP (anion). It can be concluded that the cations are not much implied, whereas the anions of PEP and PMS are hugely involved in the interactions and causes structural disruption.
4. Conclusions
The current investigation implies evidence of the destabilizing effect of PILs on collagen, witnessing the rheology of collagen to the variance in the fluorescence and absorption spectra observed for increasing concentration, with the CD spectra depicting the decrease in ellipticity values to the lower intensities of the bands in the FT-IR spectral studies and optical micrographs, which predicates slacking up of collagen. The physico-chemical properties are varied in the complete denaturation process due to the stronger interaction of the hydrogen bonds of collagen with ions resulting in a denatured state. The molecular modelling studies also reveal that the anions are responsible for the structural deformation of collagen and this was in accordance with the experimental techniques reported. Therefore, it clearly shows that the PILs interaction with collagen destabilizes the structure. In addition, further studies are underway to determine better interactions between the ionic liquids and collagen for the stabilization process, which will broaden the applications of ILs.Acknowledgements
The authors thank the CSIR–Research Initiatives for Waterless Tanning (RIWT) project for the fellowship (CSC0202). CSIR-CLRI communication code: 1178. The authors also thank Dr V. Subramanian for his insights and support towards our study. We are grateful to the C-MMACS for providing the super-computing facility used to carry out this study.Notes and references
- J. K. Fraser and D. R. MacFarlane, Aust. J. Chem., 2009, 62, 309 CrossRef.
- M. Petkovic, K. R. Seddon, L. P. N. Rebeloa and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383 RSC.
- D. Wei and A. Ivaska, Anal. Chim. Acta, 2008, 607, 126 CrossRef CAS PubMed.
- M. Moniruzzamana, K. Nakashimab, N. Kamiyaa and M. Goto, Biochem. Eng. J., 2010, 48, 295 CrossRef.
- R. A. Judge, S. Takahashi, K. L. Longnecker, E. H. Fry, C. A. Zapatero and M. L. Chiu, Cryst. Growth Des., 2009, 9, 3463 CAS.
- M. Naushad, Z. A. ALOthman, A. B. Khan and M. Ali, Int. J. Biol. Macromol., 2012, 51, 555 CrossRef CAS PubMed.
- K. J. Fraser, E. I. Izgorodina, M. Forsyth, J. L. Scott and D. R. MacFarlane, Chem. Commun., 2007, 3817 RSC.
- T. J. Wooster, K. M. Johanson, K. J. Fraser, D. R. MacFarlane and J. L. Scott, Green Chem., 2006, 8, 691 RSC.
- P. Kilaru, G. A. Baker and P. Scovazzo, J. Chem. Eng. Data, 2007, 52, 2306 CrossRef CAS.
- L. Y. Zhu, G. Q. Li and F. Y. Zheng, J. Biophys. Chem., 2011, 2, 146 CAS.
- K. Sankaranarayanan, G. Sathyaraj, B. U. Nair and A. Dhathathreyan, J. Phys. Chem. B, 2012, 116, 4175 CrossRef CAS PubMed.
- A. Kumar and P. Venkatesu, RSC Adv., 2014, 4, 4487 RSC.
- K. Fujita, D. R. MacFarlane, M. Forsyth, M. Y. Fujita, K. Murata, N. Nakamura and H. Ohno, Biomacromolecules, 2007, 8, 2080 CrossRef CAS PubMed.
- J. V. Rodrigues, V. Prosinecki, I. Marrucho, L. P. N. Rebelo and C. M. Gomes, Phys. Chem. Chem. Phys., 2011, 13, 13614 RSC.
- H. T. Karimata and N. Sugimoto, Nucleic Acids Res., 2014, 42, 8831 CrossRef PubMed.
- J. Ranke, S. Stolte, R. Stormann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183 CrossRef CAS PubMed.
- H. Zhao, J. Chem. Technol. Biotechnol., 2010, 85, 891 CrossRef CAS.
- A. Mehta, J. R. Rao and N. N. Fathima, J. Phys. Chem. B, 2015, 119, 12816 CrossRef CAS PubMed.
- A. Mehta, J. R. Rao and N. N. Fathima, Colloids Surf., B, 2014, 117, 376 CrossRef CAS PubMed.
- G. Chandrakasan, D. A. Torchia and K. A. Priez, J. Biol. Chem., 1976, 251, 6062 CAS.
- J. F. Woessner, Arch. Biochem. Biophys., 1961, 93, 440 CrossRef CAS PubMed.
- http://www.cgl.ucsf.edu./cgi-bin/gencollagen.py, The GenCollagen Database.
- H. J. C. Berendsen, D. Vander Spoel and R. van Drunen, Comput. Phys. Commun., 1995, 91, 43 CrossRef CAS.
- B. Hess, C. Kutzner, D. Vander Spoel and E. Lindahl, J. Chem. Theory Comput., 2008, 4, 435 CrossRef CAS PubMed.
- Y. Duan, C. Wu, S. Chowdhury, M. C. Lee, G. M. Xiong, W. Zhang, R. Yang, P. Cieplak, R. Luo, T. Lee, J. Caldwell, J. M. Wang and P. Kollman, J. Comput. Chem., 2003, 24, 1999 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, J. Farkas, B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A. 02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- J. Wang, W. Wang, P. Kollman and D. A. Case, J. Mol. Graphics Modell., 2006, 25, 247 CrossRef CAS PubMed.
- J. Wang, R. M. Wolf, J. W. Caldwell, P. A. Kollman and D. A. Case, J. Comput. Chem., 2004, 25, 1157 CrossRef CAS PubMed.
- W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey and M. L. Klein, J. Chem. Phys., 1983, 79, 926 CrossRef CAS.
- M. Parrinello and A. Rahman, J. Appl. Phys., 1981, 52, 7182 CrossRef CAS.
- S. Nos'e and M. L. Klein, Mol. Phys., 1983, 50, 1055 CrossRef.
- G. Bussi, D. Donadio and M. Parrinello, J. Chem. Phys., 2007, 126, 014101 CrossRef PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33 CrossRef CAS PubMed.
- C. C. Huang, G. S. Couch, E. F. Pettersen, T. E. Ferrin, A. E. Howard and T. E. Klein, Pac. Symp. Biocomput., 1998, 349 CAS.
- N. N. Fathima and A. Dhathathreyan, Int. J. Biol. Macromol., 2009, 45, 274 CrossRef CAS PubMed.
- N. O. Metrevel, K. K Jariashvili, L. O. Namicheishvilli, D. V. Svintradze, E. N. Chicvaidze and A. Sionkowsa, Ecotoxicol. Environ. Saf., 2010, 73, 448 CrossRef PubMed.
- N. J. Greenfield, Trends Anal. Chem., 1999, 18, 236 CrossRef CAS.
- S. M. Kelly, T. J. Jess and N. C. Price, Biochim. Biophys. Acta, 2005, 1751, 119–139 CrossRef CAS PubMed.
- B. C. Vidal, M. Luiza and S. Mello, Micron, 2011, 42, 283 CrossRef CAS PubMed.
- A. Barth, Biochim. Biophys. Acta, 2007, 1767, 1073 CrossRef CAS PubMed.
- A. Mehta, J. R. Rao and N. N. Fathima, Int. J. Biol. Macromol., 2014, 4346, 1 Search PubMed.
- I. Kanungo, N. N. Fathima, J. R. Rao and B. U. Nair, Mater. Chem. Phys., 2013, 140, 357 CrossRef CAS.
- I. S. Raja and N. N. Fathima, SpringerPlus, 2014, 3, 393 CrossRef PubMed.
- E. R. AzhagiyaSingam, K. Balamurugan, R. Gopalakrishnan, S. R. Subramanian, V. Subramanian and T. Ramasami, Biopolymers, 2012, 97, 847 CrossRef PubMed.
- E. Schreiner, C. Nicolini, B. Ludolph, R. Ravindra, N. Otte, A. Kohlmeyer, R. Rousseau, R. Winter and D. Marx, Phys. Rev. Lett., 2004, 92, 148101 CrossRef PubMed.
- A. M. Figueiredo, J. Sardinha, G. R. Moore and E. J. Cabrita, Phys. Chem. Chem. Phys., 2013, 15, 19632 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22441a |
| This journal is © The Royal Society of Chemistry 2016 |