Platinum nanocatalysts on metal oxide based supports for low temperature fuel cell applications
Abstract
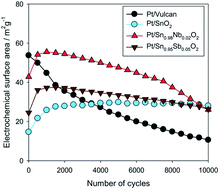
In this manuscript a survey of the contemporary research related to platinum nanocatalysts on metal oxide based supports for low temperature fuel cell applications is presented. Different carbon based supports, used as state of the art materials, are listed and discussed, as well. Although carbon based materials possess many desirable properties, such as high surface area, high conductivity and relatively low cost and easy synthesis, the large scale commercialization is limited by instability under accelerated stability testing, simulating real fuel cell operating conditions. To overcome these disadvantages of carbon supports, different metal oxide based ones have been studied and promising results are referenced. The most often used oxide based supports for low temperature fuel cell applications are presented in this review. Suitable discussion and future research related remarks are given, as well.

 Please wait while we load your content...
Please wait while we load your content...