In vivo SAR and STR analyses of alkaloids from Picrasma quassioides identify 1-hydroxymethyl-8-hydroxy-β-carboline as a novel natural angiogenesis inhibitor†
Guiyi Gong‡
a,
Qinghua Lin‡a,
Jian Xua,
Feng Yea,
Lingling Jiangb,
Wenyuan Liucd,
Ming-Fang Heb,
Feng Feng*ae,
Wei Qu*ae and
Ning Xief
aDepartment of Natural Medicinal Chemistry, China Pharmaceutical University, Nanjing 210009, China. E-mail: popoqzh@126.com; fengfeng@cpu.edu.cn; Fax: +86 25 86185216; Tel: +86 25 86185216
bInstitute of Translational Medicine College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211800, China
cDepartment of Pharmaceutical Analysis, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009, China
dKey Laboratory of Drug Quality Control and Pharmacovigilance (China Pharmaceutical University), Ministry of Education, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009, China
eKey Laboratory of Biomedical Functional Materials, China Pharmaceutical University, Nanjing 211198, China
fState Key Laboratory of Innovative Natural Medicines and TCM Injections, Jiangxi Qingfeng Pharmaceutical Co., Ltd., Ganzhou 341000, Jiangxi, China
First published on 14th January 2016
Abstract
Angiogenesis plays an important role in the development of inflammatory diseases, including cancer, psoriasis and rheumatoid arthritis. In this paper, we conducted a zebrafish bioassay-guided fractionation of Picrasma quassioides and identified twenty alkaloids from the anti-angiogenic fraction, including four new ones (1–4). In addition, in vivo relationship analyses of the structure and anti-angiogenic activity/toxicity led to the conclusion that the skeleton of alkaloids as well as the positions and properties of the substituents are pivotal to their activity and toxicity. Furancanthin (1) is the first-reported furan-fused canthin-6-one with an unprecedented highly-conjugated pentacyclic skeleton. 1-Hydroxymethyl-8-hydroxy-β-carboline (3) was found to have the most potent anti-angiogenic activity and the lowest toxicity in vivo, whose anti-angiogenic activity was also confirmed in vitro. Further qRT-PCR analysis revealed that the kdr, kdrl signaling axle in the VEGF–VEGFR pathway and the angpt2b, tek in the ANGPT–TEK pathway seemed to be involved in the anti-angiogenic activity of compound 3.
Introduction
Angiogenesis is a complex process in which several key steps are involved. Although angiogenesis is essential for normal physiological processes, it also plays an important role in the development of inflammatory diseases, including cancer, psoriasis and rheumatoid arthritis.1 Growing evidence proved that chronic inflammation and angiogenesis are codependent, involving increased cellular infiltration and proliferation as well as overlapping of regulatory growth factors and cytokines.2 Thus, anti-angiogenic strategy is emerging as an important approach for the treatment and prevention of chronic diseases.3Picrasma quassioides (D. Don) Bennet, which tastes extremely bitter, is one of the most cited species in ancient Chinese medical treatise for a wide range of indications. Its twigs and barks are registered in present Chinese and Asian pharmacopoeias. Therefore, P. quassioides is traded across China and usually prescribed as an agent in cases of acute colic of the gastrointestinal sphere with or without diarrhea. But in Nepal, the extract of P. quassioides is applied to treat some intractable skin diseases (such as psoriasis and pruritus) and has significant efficacy.4
Previous studies have shown that P. quassioides contains a diverse range of bioactive phytochemicals, including alkaloids (β-carbolines and canthin-6-ones),5–7 quassinoids8 and triterpenoids.9 Nowadays, plenty of researches showed that the alkaloids from P. quassioides manifested potent anti-inflammatory effect10–12 and attracted our attention. In addition, many anti-inflammatory herbal medicines (such as Tripterygium wilfordii13,14 and Alpinia oxyphylla15) and natural products (such as indirubin16 and rhubarb17) showed considerable anti-angiogenic activities, which might serve as promising drug candidates for the treatment of diseases with abnormally activated angiogenesis. Therefore, it is conceivable that P. quassioides and its alkaloids possess potential anti-angiogenic properties. Although β-carbolines and canthin-6-ones have been reported to have promising anti-inflammatory activities,10–12 the effect of these alkaloids on angiogenesis remains unclear. Only two recent studies touched on the anti-angiogenic effect of one β-carboline alkaloid, harmine.18,19 Moreover, it remains unclear that the relationship between the chemical structure diversity of β-carboline alkaloids and the corresponding anti-angiogenic activity.
In recent years, in vivo chemical screening in zebrafish (Tg(flia:EGFP)) has emerged as an ideal method to study anti-angiogenic substances and identify promising lead compounds.20–24 Moreover, by performing this method, researchers could not only determine the bioactivity and toxicity against the vasculature development in zebrafish embryos, but also conduct structure–activity and structure–toxicity relationships (SAR and STR) studies.25 In this report, we carried out a phytochemical investigation into the bio-active fraction and the constitutive β-carboline alkaloids, then their SAR and STR on anti-angiogenesis were also discussed using in vivo zebrafish model. Finally, the action mechanism of a novel β-carboline alkaloid, which had the best therapeutic window, was evaluated.
Results and discussion
Bioassay-guided fractionation and structure elucidation
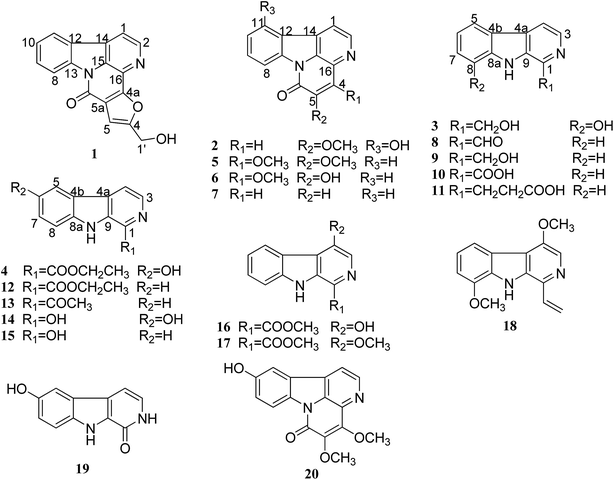
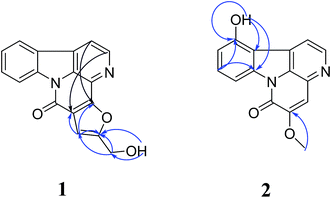
We used zebrafish as a front-line in vivo assay model for the bioassay-guided fractionation of crude extracts of P. quassioides. We excitedly found that an alkaline (pH 9) fraction which mainly contains alkaloids showed noteworthy anti-angiogenic activity (Fig. S7†), prompting us to elucidate its bio-active ingredients. We then conducted a phytochemical investigation into the bio-active fraction and obtained twenty alkaloids, including four new ones (1–4) (Fig. 1). Herein, we describe the structure elucidation of the four new compounds.Compound 1 was obtained as white floc. Its molecular formula was established as C17H10N2O3 from the positive-mode HR-ESI-MS ion at m/z 291.0767 [M + H]+ (calcd [C17H10N2O3+H]+, 291.0764), indicating 13 degrees of unsaturation. The 1H NMR spectrum (Table 1) of 1 displayed six well-splitted aromatic proton signals (δH 7.29, 7.58, 7.77, 8.28, 8.36, 8.56 and 8.84) that possess 5.7 Hz, 8.1 Hz coupling constants, one hydroxymethyl signal (δH 4.71, 2H d and 5.81, 1H t) and one olefinic proton (δH 7.29, 1H s), suggested the canthinone-type skeleton for 1. The 13C NMR spectrum (Table 1) showed 17 carbon resonances, and aided by HSQC experiments, we categorized them into one methylene (δC 56.1); seven methines, and nine quaternary carbons including three oxygenated aromatic carbons or carbonyl carbons (δC 145.2, 150.6, 163.9), collectively corresponding to the molecular formula C17H10N2O3. Then we confirmed 1 as a canthin-6-one instead of a canthin-4-one on the basis of comparison with NMR data of reported drymaritin.26 Furthermore, compared with those of 5-hydroxymethylcanthin-6-one,27 which possessed 11 degrees of unsaturation, we noticed that a downshift of olefinic proton of 1 from 8.02 to 7.29. Additionally, two other quaternary carbons and unsaturation appeared. The key HMBC correlations (Fig. 2) from CH2-1′ (δH 4.71) to C-4 (δC 163.9) and C-5a (δC 103.4), from OH (δH 5.81) to C-1′ (δC 56.1) and C-4 (δC 163.9), from olefinic proton (δH 7.29) to C-4a (δC 145.2), C-4 (δC 163.9) and C-5 (δC 132.0) revealed a hydroxymethylfuran group in 1, which might contributed to the extra quaternary carbons and unsaturation. Notably, the correlations from H-1 (δH 8.28) to C-4a (δC 145.2) and from H-2 (δH 8.84) to C-5 (δC 132.0) in HMBC experiments can lead to the presence of hydroxymethylfuran group, and the disconnection of oxygenated aromatic carbon and carbonyl carbon. Thus, 1 was determined as 4-hydroxymethyl-canthin[4,5-b]furan-6-one, named furancanthin, which is the first-reported furan-fused canthin-6-one with an unprecedented pentacyclic high-conjugated skeleton.
| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δH mult (J in Hz) | δC | HMBC (H to C) | δH mult (J in Hz) | δC | HMBC (H to C) | |
| 1 | 8.28 (1H, d, 5.1) | 115.9 | 4a, 12, 14, 15 | 7.95 (1H, d, 5.1) | 115.0 | 2, 15 |
| 2 | 8.84 (1H, d, 5.1) | 145.4 | 1, 5, 15, 16 | 8.72 (1H, d, 5.1) | 145.6 | 1, 14 |
| 4 | 163.9 | 7.40 (1H, s) | 109.9 | 6, 15, 16 | ||
| 4a | 145.2 | |||||
| 5 | 7.29 (1H, s) | 103.4 | 4, 4a, 5 | 153.5 | ||
| 5a | 132.0 | |||||
| 6 | 150.6 | 154.3 | ||||
| 8 | 8.56 (1H, d, 8.2) | 116.2 | 11, 12, 13 | 7.98 (1H, d, 8.1) | 107.0 | 12 |
| 9 | 7.77 (1H, t, 5.7) | 130.8 | 10, 11, 13 | 7.57 (1H, t, 8.1) | 131.6 | 11, 13 |
| 10 | 7.58 (1H, t, 5.7) | 125.4 | 8, 9, 12, 13 | 7.02 (1H, d, 8.2) | 111.9 | 8, 12 |
| 11 | 8.36 (1H, d, 8.2) | 123.5 | 8, 13, 14 | 154.8 | ||
| 12 | 124.8 | 112.3 | ||||
| 13 | 138.6 | 139.4 | ||||
| 14 | 130.1 | 127.4 | ||||
| 15 | 132.4 | 126.0 | ||||
| 16 | 129.6 | 135.5 | ||||
| 1′ | 4.71 (2H, d, 5.9) | 56.1 | 4, 5a | |||
| 1′-OH | 5.81 (1H, t, 6.0) | 1′, 4 | ||||
| 5-OCH3 | 3.99 (3H, s) | 56.5 | 5 | |||
| 11-OH | 11.02 (1H, s) | 11, 12, 13 | ||||
Compound 2 was isolated as yellow amorphous powder. Its HR-ESI-MS showed an [M + H]+ ion at m/z 267.0765 (calcd [C15H10N2O3+H]+, 267.0764), indicated a molecular formula of C15H10N2O3, which possesses 11 indices of hydrogen deficiency. The 1H NMR spectrum (Table 1) of 2 exhibited the resonances of one methoxy group (δH 3.99, 3H s), one reactive proton (δH 11.02, OH s), five aromatic proton (δH 7.02, 7.57, 7.95, 7.98 and 8.72) and one olefinic proton (δH 7.40, 1H s), suggesting the presence of a one-substituted carboline-type moiety. The 13C NMR (Table 1) and HSQC data revealed the presence of one methoxy (δC 56.5); six methines and eight quaternary carbons including three oxygenated aromatic carbons or carbonyl carbons (δC 153.5, 154.3, 154.8), implied a canthinone core skeleton. Compared the spectral data of 2 to those of 5-methoxycanthin-6-one,28 we easily found that they were quite similar except for a oxygenated-aromatic carbon. The correlations (Fig. 2) from OCH3 (δH 3.99) to C-5 (δC 153.5), from olefinic proton (δH 7.40) to C-6 (δC 154.3), C-15 (δC 126.0) and C-16 (δC 135.5), from OH (δH 11.02) to C-11 (δC 154.8), C-12 (δC 112.3) and C-13 (δC 139.4), from aromatic proton (δH 7.57) to C-11 (δC 154.8) and C-13 (δC 139.4) observed in HMBC confirmed the location of methoxy group was at C-5 while OH group at C-11. Therefore, the structure of 2 was determined as 11-hydroxy-5-methoxycanthin-6-one.
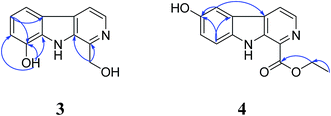
Compound 3 was obtained as brown needle. The molecular formula was established as C12H10N2O2 based on its HR-ESI-MS [M − H]− ion peak at m/z 213.0650 (calcd [C12H11N2O2 − H]−, 213.0659). The 1D NMR and HSQC spectroscopic data of 3 (Table 2) suggested its β-carboline skeleton and displayed one hydroxymethyl group, one hydroxy group. The comparison of the NMR data between 3 and 1-hydroxymethyl-β-carboline (9) revealed an extra hydroxy group in 3, which was also confirmed by its molecular formula.29 The small sample size of 3 prevented acquisition of a 13C NMR spectrum with adequate signal to noise ratio. But HMBC correlations (Fig. 3) from CH2 (δH 4.99) to C-1 (δC 145.4) and C-9 (δC 133.4), from aromatic proton (δH 6.95) to C-5 (δC 112.8), C-8a (δC 131.1) and C-8 (δC 144.2), from proton (δH 7.07) to C-4b (δC 122.7) and C-8 (δC 144.2), from OH (δH 10.05) to C-7 (δC 112.9), C-8 (δC 144.2) and C-8a (δC 131.1) suggested that the hydroxymethyl group substituted at C-1 while the hydroxy group at C-8. Thus, 3 was established as 1-hydroxymethyl-8-hydroxy-β-carboline.
| Position | 3 | 4 | ||||
|---|---|---|---|---|---|---|
| δH mult (J in Hz) | δC | HMBC (H to C) | δH mult (J in Hz) | δC | HMBC (H to C) | |
| a Chemical shifts marked with an asterisk (*) indicate overlapped signals. | ||||||
| 1 | 145.4 | 129.2 | ||||
| 3 | 8.23 (1H, d, 5.3) | 136.4 | 1, 4, 4a | 8.39 (1H, d, 4.7) | 136.6 | 1, 4, 4a, 9a |
| 4 | 8.00 (1H, d, 5.5) | 114.5 | 4b, 8 | 8.31 (1H, d, 4.6) | 118.4 | 4a, 4b, 9a |
| 4a | 129.3 | 130.1 | ||||
| 4b | 122.7 | 120.4 | ||||
| 5 | 7.67 (1H, d, 8.1) | 112.8 | 4b, 7, 8a | 7.58 (1H, s) | 105.4 | 4a, 7, 8a |
| 6 | 7.07 (1H, t, 7.8) | 120.9 | 4b, 8 | 151.1 | ||
| 7 | 6.95 (1H, d, 7.5) | 112.9 | 5, 8, 8a | 7.12 (1H, d, 8.6) | 118.6 | 5, 8a |
| 8 | 144.2 | 7.60* (1H, m) | 113.0 | 4b, 6 | ||
| 8a | 131.1 | 134.9 | ||||
| 9a | 133.4 | 136.1 | ||||
| 1′ | 4.99 (2H, d, 5.7) | 63.3 | 1, 9a | |||
| 1′-OH | 5.57 (1H, t, 5.7) | 5, 8, 8a | ||||
| 8-OH | 10.05 (1H, s) | 7, 8, 8a | ||||
| NH | 11.09 (1H, s) | 11.39 (1H, s) | 4a, 4b, 9a | |||
1′-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– O– |
165.2 | |||||
| 2′-OCH2– | 4.48 (2H, q, 7.0) | 60.4 | 1′, 3′ | |||
| 3′-CH3 | 1.41 (3H, t, 7.0) | 14.0 | 2′ | |||
Compound 4 was isolated as yellow syrup. The positive-mode HR-ESI-MS of 4 gave the ion peak at m/z 257.0919 (calcd [C14H12N2O3+H]+, 257.0921), which was consistent with the molecular formula C14H12N2O3. The 1H NMR spectrum (Table 2) displayed signals for NH at δ 11.39, besides, a set of ABX aromatic signals (δH 7.12, 1H d, 7.58, 1H m, 7.60, 1H m) indicated the presence of a mono-substituted aromatic A ring of β-carboline and the substitutes at C-6 or C-7. The ortho coupled doublets (δH 8.31 and 8.39) with smaller coupling constant of 4.7 Hz were typical of heteroaromatic protons, H-4 and H-3 of β-carboline skeleton. The proton signals (δH 1.41, 3H t and 4.48, 2H q) and three carbon signals (δC 14.0, 60.4, 165.2) (Table 2) suggested the presence of an ethoxycarbonyl group at C-1. The β-carboline ring and ethoxycarbonyl chain accounted for C14H11N2O2, with an unaccounted HO from the molecular formula C14H12N2O3. This suggested the possibility of a hydroxy moiety being the missing fragment of 4.30 Comparison of the spectral basis between 4 and 6-hydroxy-1-methoxycarbonyl-β-carboline showed the location of the OH moiety at C-6.31 In its HMBC experiments, the correlations (Fig. 3) from aromatic proton (δH 7.12) to C-5 (δC 105.4), C-8a (δC 134.9) and C-6 (δC 151.1), from proton (δH 7.58) to C-7 (δC 118.6), C-4a (δC 130.1), C-8a (δC 134.9) and C-6 (δC 151.1), from proton (δH 7.60) to C-4b (δC 120.4) and C-6 (δC 151.1) fully supported the assignments above. Hence, 4 was assigned as 6-hydroxy-1-ethoxycarbonyl-β-carboline.
The known compounds 4,5-dimethoxy-canthin-6-one (5),32 4-methoxy-5-hydroxy-canthin-6-one (6),32 canthin-6-one (7),28 1-aldehyde-β-carboline (8),33 1-hydroxymethyl-β-carboline (9),29 β-carboline-1-carboxylic acid (10),30 β-carboline-1-propionic acid (11),32 1-ethoxycarbonyl-β-carboline (12),34 1-formyl-β-carboline (13),34 1,6-dihydroxy-β-carboline (14),35 1-hydroxy-β-carboline (15),35 4-hydroxy-1-methoxycarbonyl-β-carboline (16),35 4-methoxyl-1-methoxycarbonyl-β-carboline (17),36 4,8-dimethoxy-1-vinyl-β-carboline (18),28 1-carbonyl-6-dihydroxy-β-carboline (19)35 and 4,5-dimethoxy-10-hydroxy-canthin-6-one (20)7 were identified by comparison of their observed and reported NMR data.
In vivo assessment of anti-angiogenic activity and toxicity of isolated alkaloids
Transgenic zebrafish (Tg(flia:EGFP)), which can exhibit vasculature-specific expression of enhanced green fluorescent protein (EGFP) within the whole body during embryonic and larval development, is an ideal in vivo model for studying anti-angiogenic agents.37,38 The primary advantages include their high genetic, physiologic, and pharmacologic similarity with humans, as well as the small size, optical transparency, rapid development, and large numbers of their embryos and larvae. Moreover, along with the assessment of bio-activity, the toxicity of a test compound could also be determined by the influence on the embryo development and body integrity. So, the secondary advantage of zebrafish model is that it can distinguish whether the anti-angiogenic activity of a test compound is through a direct vasculature targeting effect or through a secondary effect caused by development blocking which in turn resulted in the decrease of angiogenesis.Compounds 1–20 at various concentrations were tested for their anti-angiogenic activities and toxicities on transgenic zebrafish from 24 hpf to 72 hpf. The lowest observed effect concentration (LOEC) in terms of anti-angiogenesis, totally lethal concentration (LC100), and anti-angiogenic index (AI, AI = LC100/LOEC) values were estimated (Table 3).21,23,24,39
| Compound | Clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
LOEC (μM) | LC100 (toxicity, μM) | AI |
|---|---|---|---|---|
a Clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values: calculated from Chem3D. LOEC: lowest observed effect concentration, it was the treatment concentration that resulted in anti-angiogenic effects on most of the zebrafish larval population; LC100: totally lethal concentration, it was a concentration that resulted in 100% mortality of the embryos during the incubation period; AI: anti-angiogenic index, LC100/LOEC, was considered as the concentration range between LOEC and LC100, it revealed the sub-lethal effects of the compounds with respect to its efficacious concentrations. N.d., not determined due to precipitation. N/A, not applicable. For comparison, the effects of the known KDR inhibitor SU5416 are shown at the bottom. Results from at least 20 embryos per condition. P values: calculated from Chem3D. LOEC: lowest observed effect concentration, it was the treatment concentration that resulted in anti-angiogenic effects on most of the zebrafish larval population; LC100: totally lethal concentration, it was a concentration that resulted in 100% mortality of the embryos during the incubation period; AI: anti-angiogenic index, LC100/LOEC, was considered as the concentration range between LOEC and LC100, it revealed the sub-lethal effects of the compounds with respect to its efficacious concentrations. N.d., not determined due to precipitation. N/A, not applicable. For comparison, the effects of the known KDR inhibitor SU5416 are shown at the bottom. Results from at least 20 embryos per condition. |
||||
| 1 | 2.00 | N.d. | N.d. | N/A |
| 2 | 2.25 | >200 | >200 | 1 |
| 3 | 1.54 | 25 | 150 | 6 |
| 4 | 2.39 | 150 | 200 | 1.3 |
| 5 | 2.43 | >25 | 25 | <1 |
| 6 | 2.42 | >5 | 5 | <1 |
| 7 | 2.27 | >200 | >200 | 1 |
| 8 | 2.48 | 5 | 10 | 2 |
| 9 | 1.97 | >200 | >200 | 1 |
| 10 | 2.91 | N.d. | N.d. | N/A |
| 11 | 2.32 | >200 | >200 | 1 |
| 12 | 2.81 | 75 | 150 | 2 |
| 13 | 2.79 | >200 | >200 | 1 |
| 14 | 2.42 | >200 | >200 | 1 |
| 15 | 2.85 | 100 | >200 | 2 |
| 16 | 2.65 | 100 | 150 | 1.5 |
| 17 | 2.66 | >10 | 10 | <1 |
| 18 | 3.75 | 25 | 50 | 2 |
| 19 | 0.57 | N.d. | N.d. | N/A |
| 20 | 2.16 | >200 | >200 | 1 |
| SU5416 | 2.83 | 1 | 5 | 5 |
As the current data showed (Table 3), the structural diversity of these alkaloids and their anti-angiogenic effects/toxicity roughly defined some structure–activity/toxicity relationships. First of all, the alkaloid skeleton (β-carboline type or canthinone type) might be crucial in the determination of their structure–activity/toxicity. Most of the tested β-carbolines displayed visible anti-angiogenic effects and had satisfactory AI values while few canthin-6-ones exhibited observable phenotype. Only two canthinones (5 and 6), showing strong toxicity on zebrafish at low concentration 25 and 5 μM, respectively, inhibited angiogenisis when the larvae were dying. On the contrary, other canthinones, including 1, 2, 7, 20, manifested no anti-angiogenic effect and little toxicity to the larvae. In addition, we noticed that compound 3, 4, 8, 12, 15, 16 and 18 could exhibit different potency and effectiveness of the anti-angiogenic activities on zebrafish when the embryos were alive and with comparable body morphology and structure to those in control groups (in Table 4). All these alkaloids are β-carbolines. The survival rate of zebrafish embryos and the anti-angiogenic activity of these compounds at 72 hpf were summarized in Table 4 for better clarification. These findings suggested that canthinone-type skeleton might be inferior to β-carbolines with regard to the anti-angiogenic function.
| Number | Concentration (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 75 | 100 | 150 | 200 | |
| a The data are presented as mean ± SEM of at least three individual experiments. + is the estimated arbitrary unit of anti-angiogenic activity of the alkaloids. +, mild; ++, moderate; +++, strong. | ||||||||
| 3 | 100 | 100 | 100(+) | 100(++) | 78.3 ± 7.6(+++) | 60.0 ± 15.0(+++) | 0 | 0 |
| 4 | 100 | 100 | 100 | 100 | 100 | 93.3 ± 2.9 | 83.3 ± 2.9(+) | 0 |
| 8 | 68.3 ± 7.6(+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 100 | 100 | 100 | 100 | 98.3 ± 2.9(+) | 46.7 ± 10.4(++) | 0 | 0 |
| 15 | 100 | 100 | 100 | 100 | 100 | 100(+) | 100(++) | 100(+++) |
| 16 | 100 | 100 | 100 | 100 | 100 | 93.3 ± 7.6(+) | 0 | 0 |
| 18 | 100 | 100 | 82.7 ± 12.6(+) | 0 | 0 | 0 | 0 | 0 |
Besides, the position of substituents also exerted a tremendous influence on the activity and toxicity of these alkaloids. For example, substitutions at C-4 and C-5 in canthinones can seemingly modulate toxicity. Compound 5 and 6, which possess dual substitutions, affected the development of zebrafish embryos and appeared more toxic than the canthinones with mono-substituted or without one. Notably, compound 5 poisoned and killed the larvae at 25 μM while 20 was a safe compound for zebrafish up to 200 μM, suggesting that an additional OH group at C-10 might reduce toxicity. Similarly, the comparison of the anti-angiogenic activity of 4 with 12 (Table 4) could reveal that the substituents at position C-6 also viably resulted in decreased activity and toxicity to zebrafish, which was further confirmed by comparing 14 and 15 (Table 4). Interestingly, the observed increase in anti-angiogenic activity from 9 to 3 indicated that an OH substituent at C-8 might provide a higher chance to prevent vasculature formation.
Finally, we realized that substituents could had a drastic effect on the anti-angiogenic activity and toxicity to zebrafish. For instance, it was presumably that the hydroxy group, wherever substituted at β-carboline skeleton, could lower the toxicity to zebrafish while the replacement of a methoxy group might bring about opposite effect, as indicated by 17, 18 vs. 16. In contrast to β-carbolines, canthinone 5 was safer than 6 to zebrafish, which implied that the methoxy group at canthinones exhibited lower toxicity than hydroxy group. However, these substituents' influences on the anti-angiogenic activity remained unknown and were likely dependent on their substituted positions. From the analysis of the anti-angiogenic β-carbolines in Table 4, it could be concluded that an ester group at C-1 was possibly more active and toxic to zebrafish than carbonyl and hydroxy group, which revealed that a suitable substituent at C-1 play a key role in the anti-angiogenic activity. Compound 8 was far more toxic than other carbolines, which indicated that the aldehyde group at C-1 probably increase toxicity.
In summary, our data indicated that the alkaloid skeleton, substitutions at C-4 and C-5 (canthinones), presence of substituents at C-6 (β-carbolines) or C-10 (canthinones), variation of substituents at C-1 (β-carbolines) could significantly affect the anti-angiogenic activity and toxicity on zebrafish embryos.
Identification of a novel natural β-carboline alkaloid as a potent angiogenic inhibitor
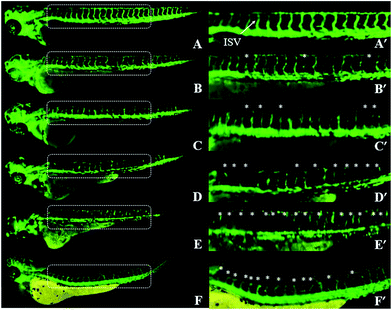
Since anti-angiogenic β-carbolines' activity and toxicity were codependent, most of their AI values approximately equal to 1. Therefore, of all investigated compounds, which conformed to the “Rule of Five”,40 the most potent anti-angiogenic activity together with the lowest toxicity to zebrafish embryos was compound 3 (Table 3). In the phenotype-based study, we found that 3 could suppress the angiogenesis in zebrafish embryos dose-dependently (Fig. 4). 3 affected the formation of ISVs with mild anti-angiogenic activity at 25 μM, and almost completely arrested the growth of ISVs at 75 μM. Due to the significant therapeutic potential of 3 in terms of its largest AI value in vivo, we subsequently investigated its anti-angiogenic activity in mammalian model.Effects of compound 3 on HUVEC proliferation, migration and tube formation in vitro
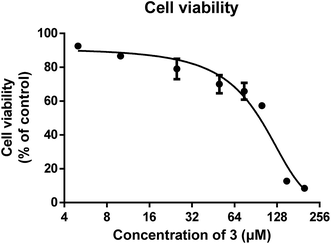
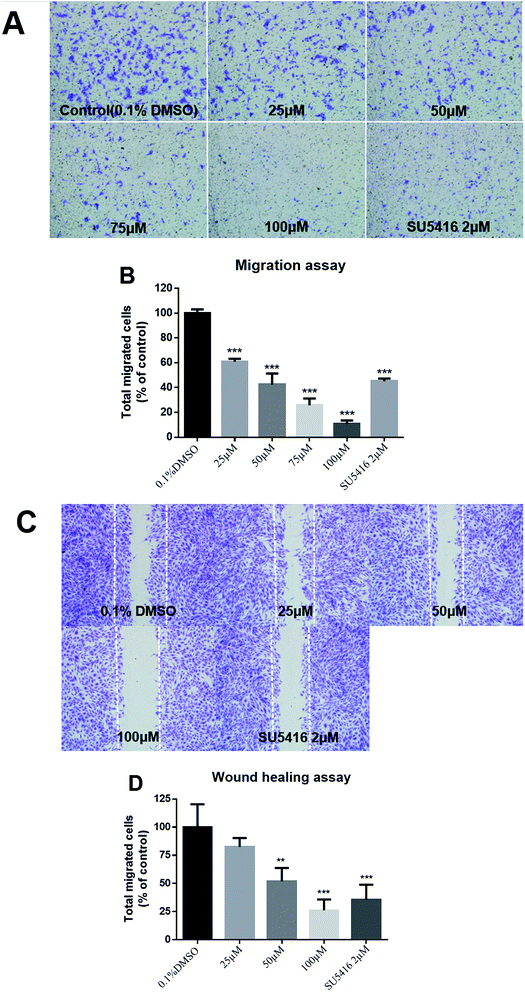
The potential anti-angiogenic effect of 3 was further verified by Human Umbilical Vein Endothelial Cell (HUVEC)-based assays in vitro. Fig. 5 shows the mean survival curves obtained with the CCK-8 assay in HUVECs, from which we estimated IC50 value was 94.2 ± 1.7 μM. This effect on cell survival was not endothelial cell-specific, since IC50 values of 3 in the treatment of several human cancer cell lines were similar to those obtained for HUVEC (results not shown).Besides, cells migration is a key step shared by both angiogenesis and tumor progression. Fig. 6A showed the effects of different concentrations of 3 on endothelial cell vertical migration ability, as determined by Boyden Chamber migration assay. Quantitative determination of the migrated cells showed a significant inhibitory effect of 3 at more than 25 μM (Fig. 6B). Additionally, 3 could inhibit HUVEC horizontal migration ability in a scratch-wound assay (Fig. 6C), achieving about 40% inhibition at 50 μM (Fig. 6D).
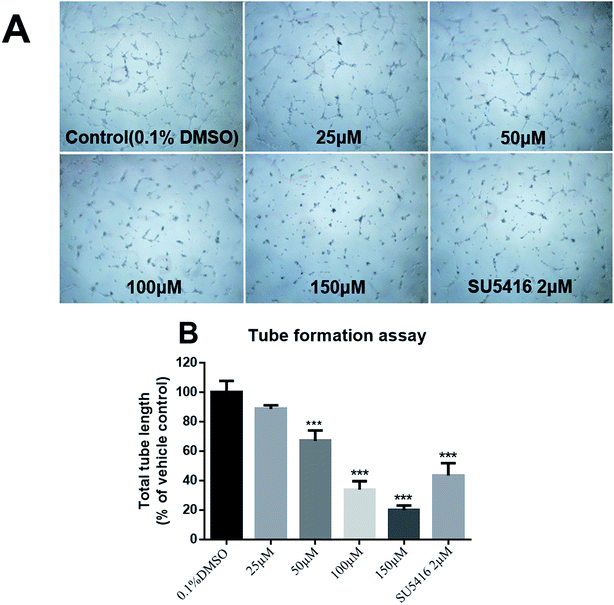
Tube formation is also indispensable for angiogenesis. The tubular structure in the treated wells was rather incomplete with fewer branch points and shorter tube length (Fig. 7A). 3 showed an inhibitory effect by about 30% and 70% at 50 μM and 100 μM respectively on HUVECs (Fig. 7B), which was comparable to the positive control.
All of the in vitro results showed that 3 could inhibit the three key steps including proliferation, migration and tube formation of HUVEC involved in the angiogenesis progress. Interestingly, 3 showed preferentially inhibition to HUVEC migration than proliferation and tube formation.
Molecular mechanism of compound 3 on zebrafish anti-angiogenesis
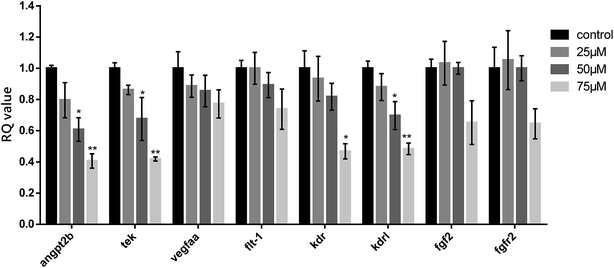
In order to explore the anti-angiogenic mechanism of compound 3, we evaluated the mRNA expression level of angiogenesis related genes (angpt2b, tek, vegfaa, flt1, kdr, kdrl, fgf2 and fgfr2) by performing qRT-PCR assay in zebrafish embryos. After 24 hours of drug treatment at 48 hpf, the expressions of these genes were indicated in Fig. 8. The mRNA expression of angpt2b and tek was consistently reduced in a dose-dependent manner (60% down-regulated at 75 μM). Besides, the expressions of two receptors in VEGF signaling pathway including kdr and kdrl were also concentration-dependently down-regulated in response to 3 treatment (50% down-regulated at 75 μM respectively). No obvious alterations of the mRNA expression level of other genes could be observed at all tested concentrations of 3. These data gave us a clue that 3 exerted anti-angiogenic actions possibly via down-regulation of both VEGF–VEGFR (kdr and kdrl) and ANGPT–TIE (angpt2b and tek) signaling pathways but not FGF/FGFR in zebrafish.Conclusion
Taken together, we conducted an in vivo zebrafish bioassay-guided fractionation approach and successfully obtained twenty alkaloids, including four new ones (1–4). In vivo phenotype-based screening of all the isolates in zebrafish identified a new alkaloid, 3 (1-hydroxymethyl-8-hydroxy-β-carboline), as a sound and effective natural angiogenesis inhibitor. The anti-angiogenic activity of 3 was confirmed by in vitro mammalian assays. Moreover, ANGPT–TIE and VEGF/VEGFR signaling pathways were found to be involved in the anti-angiogenic activity in zebrafish model.We conducted the SAR and STR studies in live zebrafish embryos, which are inherently likely to assess absorption, distribution, metabolism, excretion, and toxicity (ADMET) and identify compounds having favorable physiochemical properties and excellent “drug-likeness”. Our results demonstrate that the zebrafish model is a correlative, convenient and complementary platform for pharmaceutical development that incorporates the assessment of a lead compound's in vivo bioactivity and selectivity in the early development process.
Our findings bring new and strong evidence that β-carboline type alkaloids might serve as promising anti-angiogenic candidates. Further structural modifications of β-carbolines are still underway. Since these alkaloids' anti-inflammatory and anti-angiogenic activities were codependent, their relationships will also be explored in the future.
Experimental section
Plant material
The stems of Picrasma quassioides were collected in August 2013 from JiangXi Province, China, and identified by Prof. Feng Feng of China Pharmaceutical University. A voucher specimen (no. 130901) was deposited in the Department of Natural Medicinal Chemistry, China Pharmaceutical University, China.Animals, cell culture and chemicals
Transgenic zebrafish Tg(fli-1:EGFP) were obtained from Model Animal Research Center of Nanjing University. Primary human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Switzerland) and cultured on endothelial growth medium-2 (EGM-2, Lonza); HUVECs at early passages (passages 2–7) were used in the experiments. All cells were incubated at 37 °C in 5% CO2 (v/v). SU5416, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO).Extraction and isolation
The air-dried stems (30 kg) of P. quassioides were extracted with 95% aq. EtOH (3 × 80 L) under reflux for 2 h, and concentrated in vacuum, affording 350 g resultant aqueous residues. Then we added 3–5% hydrochloric acid solution to the extract and adjusted its pH to 1–2. After extracted with CHCl3, we added aqueous ammonia-solution and obtained pH 6, pH 9, acid-insoluble fractions by pH gradient extraction.The pH 9 fraction (45 g) was subjected to silica gel CC and eluted with CH2Cl2–MeOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–50
1–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to give 8 subfractions (9.1–9.8). Fraction 9.2 was subjected to an ODS-MPLC using a gradient elution with MeOH/H2O (10
50) to give 8 subfractions (9.1–9.8). Fraction 9.2 was subjected to an ODS-MPLC using a gradient elution with MeOH/H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90–100
90–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to afford four fractions, and the fourth fraction 9.2.4 was further separated on RP-TLC (CHCl3/MeOH 20
0) to afford four fractions, and the fourth fraction 9.2.4 was further separated on RP-TLC (CHCl3/MeOH 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get 18 (4 mg), 20 (3 mg). Purification of fraction 9.3 on Sephadex LH-20 CC using an isocratic elution with CHCl3/MeOH (1
1) to get 18 (4 mg), 20 (3 mg). Purification of fraction 9.3 on Sephadex LH-20 CC using an isocratic elution with CHCl3/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 9 (30 mg), 14 (2 mg), 15 (2 mg), 19 (30 mg). Subfraction 9.4 was further purified by repeated silica gel CC and 1 (4.3 mg), 2 (3 mg), 5 (>1 g), 8 (20 mg), 10 (6 mg), 16 (20 mg), 6 (>2 g) was obtained by recrystallized. Fraction 9.5 was subjected to a silica gel CC using a step gradient of CHCl3/MeOH (30
1) to yield 9 (30 mg), 14 (2 mg), 15 (2 mg), 19 (30 mg). Subfraction 9.4 was further purified by repeated silica gel CC and 1 (4.3 mg), 2 (3 mg), 5 (>1 g), 8 (20 mg), 10 (6 mg), 16 (20 mg), 6 (>2 g) was obtained by recrystallized. Fraction 9.5 was subjected to a silica gel CC using a step gradient of CHCl3/MeOH (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–8
1–8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent to furnish five fractions, and the fifth fraction 9.5.5 was purified by Sephadex LH-20 CC eluted with isocratic CHCl3/MeOH (1
2) as eluent to furnish five fractions, and the fifth fraction 9.5.5 was purified by Sephadex LH-20 CC eluted with isocratic CHCl3/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 3 (1 mg). The fraction 9.5.4 was purified on semi-RP-HPLC (60% MeOH, 2 mL min−1) to yield compounds 4 (5 mg), 13 (3 mg). Subfraction 9.6 (5 g) was repeatedly chromatographed over MCI gel (MeOH/H2O 1
1) to give compound 3 (1 mg). The fraction 9.5.4 was purified on semi-RP-HPLC (60% MeOH, 2 mL min−1) to yield compounds 4 (5 mg), 13 (3 mg). Subfraction 9.6 (5 g) was repeatedly chromatographed over MCI gel (MeOH/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–7
9–7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), silica gel (CH2Cl2/actone 10
3), silica gel (CH2Cl2/actone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–6
1–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Sephadex LH-20 (CH2Cl2/MeOH 1
1), Sephadex LH-20 (CH2Cl2/MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ODS (MeOH/water 3
1), ODS (MeOH/water 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7–5
7–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford 7 (2.5 mg), 11 (6.3 mg), 12 (8 mg), 17 (5 mg). The purity of compounds 1–20 was estimated to be greater than 90%, as determined by 1H NMR spectra.
5) to afford 7 (2.5 mg), 11 (6.3 mg), 12 (8 mg), 17 (5 mg). The purity of compounds 1–20 was estimated to be greater than 90%, as determined by 1H NMR spectra.
Anti-angiogenic screening of phytochemicals in zebrafish model
Adult zebrafish were kept and raised according to the zebrafish book guidelines. We used 20 dechorinated 24 hpf-embryos per experimental group in a 24-well plate and incubated with 1 mL of embryo water containing various concentrations of drug at 28.5 °C for additional 48 h. In order to evaluate the compounds' activities in a fast and efficient way, we tested all the compounds at doses of 1, 5, 10, 25, 50, 75, 100, 150, 200 μM. After anesthetized with 0.016% tricaine (Sigma-Aldrich), the inter-segmental blood vessels (ISVs) of embryos were observed and imaged at 72 hpf under a fluorescence microscope (IX71, Olympus, Japan). SU5416 was served as a positive control. All zebrafish studies were approved by the Institutional Animal Care and Use Committee at Nanjing Tech University.In vitro proliferation, migration, tube formation assays
All the assays in HUVECs were done following the methods reported previously with some modifications and were described in detail in the ESI.†41,42Reverse transcription, and quantitative real-time PCR
Zebrafish embryos at 24 hpf were treated with 0.1% DMSO and various concentrations of 3 for 24 h. At 48 hpf, total RNA was extracted from 10 zebrafish embryos per treatment group using Trizol Reagent (Invitrogen, USA) according to the manufacturer's protocol. qRT-PCR was performed on the ABI 7900HT Real-Time PCR System (Applied Biosystems, USA) using Taqman probe according to the manufacturer's instructions. The abundance of mRNA was normalized to gapdh levels and expressed as percentage of the control (100%) for statistical analysis. The primers were specific for gapdh (GenBank ID AF057040), angpt2b (AF379603), tek (AF053632), vegfaa (AF059661), flt1 (BC163921), kdr (DQ026829), kdrl (AY056466), fgf2 (AY269790) and fgfr2 (AJ309303).Statistical analysis
All experiments were repeated at least three times. Values are given as means ± the SEM. Data were analyzed using GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA). Statistical significance was assessed by one-way analysis of variance (ANOVA) or Student's t test. P values less than 0.05 were considered statistically significant.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (No. 81373956, No. 81274064, No. 81502950 and No. 81573557), the Fundamental Research Funds for the Central Universities (2015ZD010) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- J. Folkman, Nat. Med., 1995, 1, 27–30 CrossRef CAS PubMed.
- D. H. Paper, Planta Med., 1998, 64, 686 CrossRef CAS PubMed.
- P. Carmeliet, Nat. Med., 2003, 9, 653–660 CrossRef CAS PubMed.
- N. P. Manandhar, Plants and people of Nepal, Timber Press, Portland, 2002 Search PubMed.
- W. H. Jiao, G. D. Chen, H. Gao, J. Li, B. B. Gu, T. T. Xu, H. B. Yu, G. H. Shi, F. Yang, X. S. Yao and H. W. Lin, J. Nat. Prod., 2015, 78, 125–130 CrossRef PubMed.
- W. H. Jiao, H. Gao, C. Y. Li, F. Zhao, R. W. Jiang, Y. Wang, G. X. Zhou and X. S. Yao, J. Nat. Prod., 2010, 73, 167–171 CrossRef CAS PubMed.
- M. X. Jiang and Y. J. Zhou, J. Asian Nat. Prod. Res., 2008, 10, 1009–1012 CrossRef PubMed.
- S. P. Yang and J. M. Yue, Helv. Chim. Acta, 2004, 87, 1591–1600 CrossRef CAS.
- Y. Niimi, H. Hirota, T. Tsuyuki and T. Takahashi, Chem. Pharm. Bull., 1989, 37, 57–60 CAS.
- W. H. Jiao, H. Gao, F. Zhao, H. W. Lin, Y. M. Pan, G. X. Zhou and X. S. Yao, Chem. Pharm. Bull., 2011, 59, 359–364 CrossRef CAS PubMed.
- H. Fan, D. Qi, M. Yang, H. Fang, K. Liu and F. Zhao, Phytomedicine, 2013, 20, 319–323 CrossRef CAS PubMed.
- F. Zhao, Z. T. Gao, W. H. Jiao, L. P. Chen, L. Chen and X. S. Yao, Planta Med., 2012, 78, 1906–1911 CrossRef CAS PubMed.
- M. F. He, L. Liu, W. Ge, P. C. Shaw, R. Jiang, L. W. Wu and P. P. But, J. Ethnopharmacol., 2009, 121, 61–68 CrossRef CAS PubMed.
- M. F. He, Y. H. Huang, L. W. Wu, W. Ge, P. C. Shaw and P. P. But, Int. J. Cancer, 2010, 126, 266–278 CrossRef CAS PubMed.
- Z. H. He, W. Ge, G. G. Yue, C. B. Lau, M. F. He and P. P. But, J. Ethnopharmacol., 2010, 132, 443–449 CrossRef PubMed.
- D. Alex, I. K. Lam, Z. Lin and S. M. Lee, J. Ethnopharmacol., 2010, 131, 242–247 CrossRef CAS PubMed.
- Z. H. He, M. F. He, S. C. Ma and P. P. But, J. Ethnopharmacol., 2009, 121, 313–317 CrossRef CAS PubMed.
- F. Dai, Y. Chen, Y. Song, L. Huang, D. Zhai, Y. Dong, L. Lai, T. Zhang, D. Li, X. Pang, M. Liu and Z. Yi, PLoS One, 2012, 7, e52162 CAS.
- T. P. Hamsa and G. Kuttan, Eur. J. Pharmacol., 2010, 649, 64–73 CrossRef CAS PubMed.
- L. I. Zon and R. T. Peterson, Nat. Rev. Drug Discovery, 2005, 4, 35–44 CrossRef CAS PubMed.
- J. Hao, J. N. Ho, J. A. Lewis, K. A. Karim, R. N. Daniels, P. R. Gentry, C. R. Hopkins, C. W. Lindsley and C. C. Hong, ACS Chem. Biol., 2010, 5, 245–253 CrossRef CAS PubMed.
- T. V. Bowman and L. I. Zon, ACS Chem. Biol., 2010, 5, 159–161 CrossRef CAS PubMed.
- A. Papakyriakou, P. Kefalos, P. Sarantis, C. Tsiamantas, K. P. Xanthopoulos, D. Vourloumis and D. Beis, Assay Drug Dev. Technol., 2014, 12, 527–535 CrossRef CAS PubMed.
- G. Chimote, J. Sreenivasan, N. Pawar, J. Subramanian, H. Sivaramakrishnan and S. Sharma, Drug Des., Dev. Ther., 2014, 8, 1107–1123 CrossRef CAS PubMed.
- Y. Peng, J. Li, Y. Sun, J. Y. W. Chan, D. Sheng, K. Wang, P. Wei, P. Ouyang, D. Wang, S. M. Y. Lee and G. C. Zhou, RSC Adv., 2015, 5, 22510–22526 RSC.
- I. Wetzel, L. Allmendinger and F. Bracher, J. Nat. Prod., 2009, 72, 1908–1910 CrossRef CAS PubMed.
- T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1984, 32, 170–173 CrossRef CAS.
- T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1983, 31, 3198–3204 CrossRef CAS.
- Y. Kondo and T. Takemoto, Chem. Pharm. Bull., 1973, 21, 837–839 CrossRef CAS.
- Z. Q. Lai, W. H. Liu, S. P. Ip, H. J. Liao, Y. Y. Yi, Z. Qin, X. P. Lai, Z. R. Su and Z. X. Lin, Chem. Nat. Compd., 2014, 50, 884–888 CrossRef CAS.
- P. L. Wu, F. W. Lin, T. S. Wu, C. S. Kuoh, K. H. Lee and S. J. Lee, Chem. Pharm. Bull., 2004, 52, 345–349 CrossRef CAS PubMed.
- T. Ohmoto and K. Koike, Chem. Pharm. Bull., 1984, 32, 3579–3583 CrossRef CAS.
- M. Chen, H. Y. Fan, S. J. Dai and K. Liu, Chin. Tradit. Herb. Drugs, 2007, 38, 807–810 CAS.
- J. S. Yang and S. R. Luo, Acta Pharm. Sin., 1979, 14, 167–177 CAS.
- W. H. Jiao, H. Gao, C. Y. Li, G. X. Zhou, S. Kitanaka, A. Ohmura and X. S. Yao, Magn. Reson. Chem., 2010, 48, 490–495 CAS.
- T. Ohmoto, R. Tanaka and T. Nikaido, Chem. Pharm. Bull., 1976, 24, 1532–1536 CrossRef CAS.
- N. D. Lawson and B. M. Weinstein, Dev. Biol., 2002, 248, 307–318 CrossRef CAS PubMed.
- A. D. Crawford, C. V. Esguerra and P. A. de Witte, Planta Med., 2008, 74, 624–632 CrossRef CAS PubMed.
- I. K. Lam, D. Alex, Y. H. Wang, P. Liu, A. L. Liu, G. H. Du and S. M. Lee, Mol. Nutr. Food Res., 2012, 56, 945–956 CAS.
- J. B. Veselinovic, G. M. Kocic, A. Pavic, J. Nikodinovic-Runic, L. Senerovic, G. M. Nikolic and A. M. Veselinovic, Chem.-Biol. Interact., 2015, 231, 10–17 CrossRef CAS PubMed.
- M. F. He, X. P. Gao, S. C. Li, Z. H. He, N. Chen, Y. B. Wang and J. X. She, Eur. J. Pharmacol., 2014, 740, 240–247 CrossRef CAS PubMed.
- V. Iman, H. Karimian, S. Mohan, Y. H. Hobani, M. I. Noordin, M. R. Mustafa and S. M. Noor, Drug Des., Dev. Ther., 2015, 9, 1281–1292 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Extensive experimental methods, 1D NMR, 2D NMR, HRESIMS spectra of 1–4 and bioassay-guided fractionation results. See DOI: 10.1039/c5ra22391a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |