Isocyanide-based three component reaction for synthesis of highly cyano substituted furan derivatives†
Hamid Reza Safaei* and
Farbod Dehbozorgi
Department of Applied Chemistry, Faculty of Science, Shiraz Branch, Islamic Azad University, P.O. Box 71993-5, Shiraz, Iran. E-mail: safaei@iaushiraz.ac.ir
First published on 4th March 2016
Abstract
A rapid and efficient solvent-free one-pot synthesis of novel alkyl amino aryl furan tricarbonitrile derivatives is described under catalyst-free conditions. This reaction was carried out through a three-component condensation reaction of isocyanides, 2-arylidenemalononitriles and benzoyl cyanide under solvent-free conditions. Neat conditions were established with many advantages, including high yield, environmentally friendliness, and easy and quick isolation of the products. The newly synthesized compounds were systematically characterized by IR, 1H NMR, 13C NMR and elemental analysis.
1. Introduction
Nowadays, the pace of development of technology is becoming faster each year, especially in chemical fields. In this regard, organic chemists are facing new challenges for producing novel compounds that are structurally more diverse and prepared more efficiently. To achieve this goal, chemistry researchers' main concern is to have more innovative green methods that lead to new and more complex materials without environmental impact.1 Among synthetic methods, multicomponent reactions (MCRs) are currently attracting attention of organic chemists due to the new synthetic applications in agreement with green chemistry principles.2 A wide range of advantages offered by multicomponent reactions. These reactions are used to synthesize target compounds with high degree of convergence and atom economy. They generate structural complexity in a single operation without isolation of intermediates from three or more reactants.3There is no doubt isocyanides are indispensable constituents of MCRs. In the past decades, they were used in many cycloaddition reactions and proved themselves to be irreplaceable building blocks in modern multi-component chemistry because they are able to react with both nucleophiles and electrophiles at the same carbon.4,5
In recent years, more sever legislation and restrictions are decreed in order to avoid or at least, reduce of the environmental impact of manmade chemicals. In this regard, the role of solvent as reaction media to provide the requirements of environmental sustainability is inevitable. It has also been said that ‘the best solvent is no solvent’.6 Avoiding the use of solvents in synthesis of chemical compounds can reduce environmental contamination and promise to be an essential facet of ‘Green Chemistry’. They can even be more convenient than using solvent-based synthesis. Solvent-free reactions have attracted much interest because of their ease of experimental procedures and workup, low cost and environmentally benign nature.7
Without doubt, heterocyclic compounds play important roles in the drug discovery process. Functionalized heterocycles are present in a wide variety of drugs, vitamins, natural products, biomolecules and biologically active compounds. Moreover, they have been frequently found as a key structural unit in synthetic pharmaceuticals and agrochemicals. Furthermore, some of polycyano heterocyclic compounds exhibit a significant solvatochromic and photochromic properties.8 Interesting color changing property of dye chromophores with different solvents named solvatochromism effect.9 These polycyano heterocycles have attracted much attention due to optical emission properties such as photoluminescence (PL) and electroluminescence (EL), which can be used in the dye lasers10,11 and sensors.12 The most favorable photoactive dyes are merocyanines. They enable a convenient investigation of polyene–polymethine transformations in their chromophore because of their chemical structures that consists of both electron-donor and electron-acceptor groups (Fig. 1).8,13
In most of these dyes, tricyanovinyldihydrofuran derivatives (Fig. 1-2) play important roles as an acceptor part of chromophores.14
Over a hundred year ago the adduct of active methylene compounds and carbonyl groups was obtained by Emil Knoevenagel.15 This pioneering work of Knoevenagel have made impressive advances in the synthesis of five and six membered homo and hetero-cyclic compounds.16,17 Recently, Takahashi18 and Nair19 have subsequently reported the formation of dicynocyclopentene derivatives from intermolecular coupling of arylidenemalononitriles, alkynes and isocyanates or isocyanides, respectively. Later, dicyano-2,3-dihydro-1H-indene derivatives were synthesized via three-component reaction of 2-alkynylbenzaldehyde, malononitrile, and indole.20 It has been known from the studies of various groups that the 1,3 zwitterionic intermediate generated by the reaction of alkyl isocynides and dimethyl acetylenedicarboxylate.21
The success of these reactions and considering the literature background that was given above, about reactivity of Knoevenagel's adducts and in view of our general interest to isocyanide-based multicomponent reaction,22 provided us with a conceptual framework for designing novel multicomponent reactions (MCRs). So, as part of our ongoing program to develop new, efficient, and more environmentally benign methods for organic synthesis,23 we herein report a facile, environmentally benign isocyanides based one-pot three-component strategy for the synthesis of novel highly cyano substituted furan derivatives in solvent less condition (Scheme 1).
2. Results and discussion
According to Nair's21 and Bazgir's24 reports (Scheme 2A) that their product formation were mechanistically proposed through the initial formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 zwitterionic intermediate 1 between isocyanide and DMAD, and due to many other researches25–27 that in all of these reports the obtained product formation were explained through the involvement of 1,3-dipolar intermediate 2 as shown in the Scheme 2, we initiated our studies with the three-component reaction of 4-nitrobenzaldehyde (1 mmol), 2-benzylidenemalononitrile (1 mmol) and cyclohexyl isocyanide (1 mmol) in dry benzene at 80 °C to afford compound 8 or 8′ (Scheme 2B).
1 zwitterionic intermediate 1 between isocyanide and DMAD, and due to many other researches25–27 that in all of these reports the obtained product formation were explained through the involvement of 1,3-dipolar intermediate 2 as shown in the Scheme 2, we initiated our studies with the three-component reaction of 4-nitrobenzaldehyde (1 mmol), 2-benzylidenemalononitrile (1 mmol) and cyclohexyl isocyanide (1 mmol) in dry benzene at 80 °C to afford compound 8 or 8′ (Scheme 2B).
Unluckily, the desired product 8 or 8′ was not obtained, even under inert atmosphere and after extended reaction time up to 16 h. Examination of the reaction with different aldehydes such as benzaldehyde and p-methoxybenzaldehyde have not improved the reaction. It is known that the solvent plays a crucial factor for organic reactions; so, the reaction was performed in the various solvents but there was no breakthrough in progress of the reaction.
Recently, Teimouri has developed one-pot three component condensation reaction which afforded a diverse array of 9 in the absence of catalyst28 (Scheme 3C).
So, we investigated the one pot three component condensation reaction of cyclohexyl isocyanide 3a, 2-benzylidenemalononitrile 4a and benzoyl cyanide 5 in dry benzene at 80 °C to for 4 h without any additive. To our delight, it afforded highly cyano substituted 5-(cyclohexylamino)-2,4-diphenylfuran-2,3,3(2H)-tricarbonitrile 6aa in 80% isolated yield (Scheme 3D). Encouraged by this success, we examined this reaction in various mediums and temperatures. The results are listed in Table 1. Only a trace amount of the product was detected in THF, DMF, EtOH and toluene, (entries 1–3 and 7). Although water was proved to be capable of promoting the reaction, a huge improvement was observed in benzene (entry 5). Moreover, in water a gummy solid was obtained which makes the separation of products very difficult. Then we investigated the efficiency of benzene as a solvent in lower temperature. At this condition the reaction proceeded with very low yield (entry 6). Interestingly, the reaction has shown the best result in neat conditions (entry 8) and the purity of the obtained product was very high. At this condition, decreasing the temperature lowered the reaction yield (entry 9).
With these results in hand, we screened the substrate scope of the synthesis of 5-(alkylamino)-4-(aryl)-2-phenylfuran-2,3,3(2H)-tricarbonitrile in neat conditions, and the results are listed in Table 2.
| Product | R | X | Yielda (%) | Mp (°C) |
|---|---|---|---|---|
| a Isolated yields. | ||||
| 6aa | C6H11 | H | 90 | 210–212 |
| 6ba | (CH3)3C | H | 90 | 217–219 |
| 6ca | 4-Me-C6H4-SO2-CH2 | H | 91 | 222–224 |
| 6ab | C6H11 | 4-NO2 | 87 | 241–243 |
| 6bb | (CH3)3C | 4-NO2 | 90 | 238–240 |
| 6cb | 4-Me-C6H4-SO2-CH2 | 4-NO2 | 92 | 260–261 |
| 6ac | C6H11 | 4-Cl | 84 | 230–232 |
| 6bc | (CH3)3C | 4-Cl | 82 | 218–220 |
| 6cc | 4-Me-C6H4-SO2-CH2 | 4-Cl | 87 | 235–237 |
| 6ad | C6H11 | 4-MeO | 56 | 226–228 |
| 6bd | (CH3)3C | 4-MeO | 43 | 216–218 |
| 6cd | 4-Me-C6H4-SO2-CH2 | 4-MeO | 63 | 236–238 |
| 6ae | C6H11 | 4 F | 27 | 221–223 |
| 6be | (CH3)3C | 4 F | 25 | 213–214 |
| 6ce | 4-Me-C6H4-SO2-CH2 | 4 F | 30 | 227–229 |
As it is shown in Table 2, all reactions proceeded efficiently and the desired products were produced in good yields. 2-(4-Subsituated benzylidene) malononitrile with electron withdrawing groups (Table 2; 6ab–6cc) reacted more efficiently compared with those with electron releasing groups (Table 2; 6ad–6cd). While, our methodology has not been worked properly for fluorine substituted of compound 4 (Table 2; 6ae–6ce) the NMR spectroscopy of the crude products of clearly indicated that there were no products other than 6 were formed. The structures of the products 6aa–6ce were deduced from IR, 1H and 13C NMR, mass spectral data and elemental analysis. The mass spectra of these compounds displayed molecular ion peaks at the appropriate m/z values. The IR spectrum of 6aa showed strong absorption at 3422 related to R–NH side chain of furan moiety and at 2374 due to the cyano groups. The 1H NMR spectrum of 6aa exhibited three multiplet signals readily recognized as arising from cyclohexyl ring and aromatic protons at δ 1.17–1.87, 3.25 and δ 7.21–7.75 ppm respectively, and R–NH gave rise as a singlet peak at δ 8.48 ppm. The 13C NMR spectrum displayed nineteen distinct resonances consistent suggested structure. The characteristic signals due to the three carbons of cyano groups were described at δ 108.29, 108.30 and 117.56 ppm. The carbon of furan ring (C(CN)2) resonated at δ 36.82 ppm. Partial assignment of these resonances is given in the experimental data.
Furthermore, the assignment of the proton resonance with the structure of 6aa was also confirmed by a homonuclear correlation spectroscopy (COSY) 2D-NMR spectrum shown in Fig. 2a, which provided 1H–1H connectivity of neighboring protons, showing a correlation between H3a and H3b. This correlation is in good agreement with the labelled 6aa structure in Fig. 2a. Moreover, the spectrum of J-modulated spin-echo experiment was shown negative amplitudes for C1a–c and C2a–c aromatic and C3a related to cyclohexyl that in compliance with structure 6aa (Fig. 2b).
 | ||
| Fig. 2 Nuclear magnetic resonance spectra (300 MHz, CDCl3) for 6aa: (a) 2D-homonuclear correlation spectroscopy (COSY) (b) spectrum of J-modulated spin-echo experiment. | ||
The 1H and 13C NMR spectra of 6ab–6ce are similar to 6aa, except for isocyanides, and aryl moiety, and the results are reported in the Experimental section.
Although we have not established the mechanism of the reaction, on the basis of the reaction of isocyanides and adducts of Knoevenagel reaction25–27 it is reasonable to assume that compound 6 was formed by initial formation of a highly reactive 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 zwitterionic adduct 10 via nucleophilic attack of isocyanides 3 to arylidine malonitrile 4. Then this intermediate was added to carbonyl group of benzoyl cyanide 5 and dipolar species 11 was formed.
1 zwitterionic adduct 10 via nucleophilic attack of isocyanides 3 to arylidine malonitrile 4. Then this intermediate was added to carbonyl group of benzoyl cyanide 5 and dipolar species 11 was formed.
Afterward, cyclization reaction was occurred and follow with hydrogen exchange leads to the alkyl amino aryl furan tricarbonitrile derivatives 6 (Scheme 4). Although we have not concerned the stereochemistry of products, it is worth mentioning that before cyclization the adduct 11 can be rotated around (CN)(ph)C–C(CN)2 bond and the other conformer 11′ is formed (Scheme 4). Then, each of these two conformer can produce different stereoisomers 6 and 6′ through cyclization and trace with hydrogen exchange.
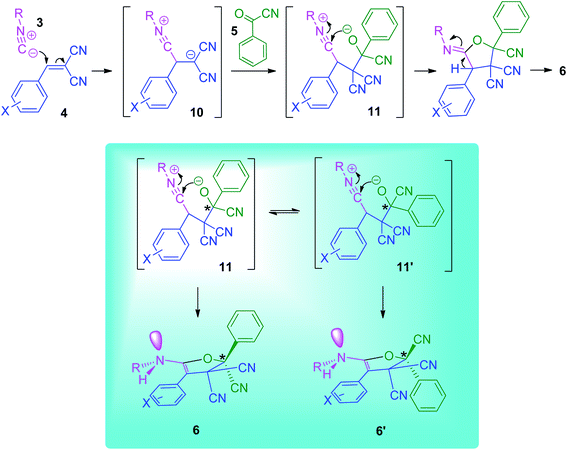 | ||
| Scheme 4 Proposed mechanism for the synthesis of the alkylamino aryl furan tricarbonitrile derivatives. | ||
In conclusion, we have developed a clean, efficient and simple one-pot three component reaction of isocyanides, 2-aryl malonate and benzoyl cyanides for the synthesis of highly cyano substituted furan derivatives 6 in solvent less conditions. Moreover, in present method not only does not need to toxic solvent and any additive or activating agent as promoter of reaction but the reaction carries in neutral conditions and no need to any special condition such as inert atmosphere or exclusion of moisture from reaction medium.
3. Experimental
3.1. General
Reagents and solvents were purchased from Merck, Fluka or Aldrich and were used without further purification. Melting points were determined in capillary tubes in an Electrothermal 9100 apparatus and uncorrected. The progress of the reaction and the purity of compounds were monitored by TLC analytical silica gel plates (Merck 60 F250). IR spectra: Perkin-Elmer spectrum RXI. FT-IR apparatus. The 1H NMR (400 MHz) and 13C NMR (100 MHz) were run on a Bruker Avance DPX-250, FT-NMR spectrometer. Chemical shifts are given as δ values against tetramethylsylane as internal standard and J values are given in Hz. Microanalysis was performed on a Perkin-Elmer 240 B microanalyzer.3.2. General procedure for preparation of 2-arylidenemalononitriles (4)
2-Arylidenemalononitriles were prepared via the method that have been reported previously29 with a little manipulation. For preparation of 4a–e In a round-bottomed flask tetra butyl ammonium bromide (TBAB) (0.08 g, 5 mol%) was added to a solution of aldehyde (5 mmol) and malononitrile (5 mmol) in 5 mL of distilled water. The mixture was stirred at room temperature for 1–2 h until complete disappearance of the aldehyde. Then, allowed to stand overnight. Afterwards, the products 4 were collected by suction filtration, washed with water and petroleum ether, dried at room temperature and the products were recrystallized using hexane to give 2-arylidenemalononitriles. The structure of products were determined by comparison of their physical and spectroscopic data with reported compounds.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic), 1617 (C
C aromatic), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 2223 (C
C), 2223 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2361 (CN). 1H NMR (400 MHz, DMSO-d6): δH 7.20–7.35 (5H, m, CH aromatic), 7.84 (1H, s, CH). 13C NMR (100 MHz, DMSO-d6): δC 82.37 (CN–C–CN), 113.31 (C
N), 2361 (CN). 1H NMR (400 MHz, DMSO-d6): δH 7.20–7.35 (5H, m, CH aromatic), 7.84 (1H, s, CH). 13C NMR (100 MHz, DMSO-d6): δC 82.37 (CN–C–CN), 113.31 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 114.09 (C
N), 114.09 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 129.15, 129.61, 130.72, 131.14 (C aromatic), 160.51 (CH).
N), 129.15, 129.61, 130.72, 131.14 (C aromatic), 160.51 (CH).3.3. General procedure for preparation of 5-(cyclohexylamino)-2,4-diphenylfuran-2,3,3(2H)-tricarbonitrile (6aa)
To a magnetically stirred mixture of benzoyl cyanide (0.131 g, 1.0 mmol) and 2-benzylidenemalononitrile (0.154 g, 1.0 mmol) was added slowly cyclohexyl isocyanide (0.109 g, 1.0 mmol) and heating was continued for 4 h. The reaction progress was monitored by TLC (EtOAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane 3
n-hexane 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then after cooling to room temperature, diethyl tether was added to crud mixture and cooled to −5 °C. Afterwards, the product was collected by suction filtration, washed with diethyl ether to afford the pure product 6aa: (0.355 g, 90%) as white powder. Mp 210–212 °C. IR (KBr) (νmax, cm−1): 1381 (C–O), 2374 (CN), 3422 (NH). 1H NMR (DMSO-d6, 400 MHz): δH 1.17–1.87, (10H, m, 5CH2, cyclohexyl), 3.25 (1H, s, CH–N), 7.21–7.75 (10H, m, CH aromatic), 8.48 (1H, s, NH). 13C NMR (100 MHz, DMSO-d6): δC 25.34, 26.04, 32.46, (CH2, cyclohexyl), 36.82 (C(CN)2), 52.31 (C–H, cyclohexyl), 82.03 (C–Ar), 87.12 (C–O), 108.29, 108.30, 117.56 (CN), 127.61, 127.85, 128.58, 128.81, 128.99, 129.12, 130.97, 135.18 (C aromatic), 137.40 (O–C–N). Anal. calcd for C25H22N4O (394.47): C, 76.12; H, 5.62; N, 14.20%. Found: C, 76.19; H, 5.51; N, 14.18%.
1). Then after cooling to room temperature, diethyl tether was added to crud mixture and cooled to −5 °C. Afterwards, the product was collected by suction filtration, washed with diethyl ether to afford the pure product 6aa: (0.355 g, 90%) as white powder. Mp 210–212 °C. IR (KBr) (νmax, cm−1): 1381 (C–O), 2374 (CN), 3422 (NH). 1H NMR (DMSO-d6, 400 MHz): δH 1.17–1.87, (10H, m, 5CH2, cyclohexyl), 3.25 (1H, s, CH–N), 7.21–7.75 (10H, m, CH aromatic), 8.48 (1H, s, NH). 13C NMR (100 MHz, DMSO-d6): δC 25.34, 26.04, 32.46, (CH2, cyclohexyl), 36.82 (C(CN)2), 52.31 (C–H, cyclohexyl), 82.03 (C–Ar), 87.12 (C–O), 108.29, 108.30, 117.56 (CN), 127.61, 127.85, 128.58, 128.81, 128.99, 129.12, 130.97, 135.18 (C aromatic), 137.40 (O–C–N). Anal. calcd for C25H22N4O (394.47): C, 76.12; H, 5.62; N, 14.20%. Found: C, 76.19; H, 5.51; N, 14.18%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2360 (CN), 3415 (NH). 1H NMR (DMSO-d6, 400 MHz): δH 2.40 (3H, s, Me), 3.80 (s, CH3–O), 4.22 (1H, d, J = 7.65, N–CH2–S), 4.67 (1H, d, J = 7.65, N–CH2–S), 7.30–8.17 (14H, m, CH aromatic), 8.94 (1H, s, NH). 13C NMR (100 MHz, DMSO-d6): δC 21.62 (CH3-ph), 36.82 (C(CN)2), 57.35 (CH3 methoxy), 61.38 (N–CH2–S), 80.02 (C–Ar), 87.95 (C–O), 108.31, 117.56 (CN), 124.85, 126.25, 127.51, 128.23, 128.43, 128.66, 129.07, 132.83, 133.31, 134.01 (C aromatic), 136.97 (C–S), 137.02 (O–C–N). Anal. calcd for C28H22N4O4S (510.56): C, 65.87; H, 4.34; N, 10.97%. Found: C, 65.81; H, 4.37; N, 10.95%.
O), 2360 (CN), 3415 (NH). 1H NMR (DMSO-d6, 400 MHz): δH 2.40 (3H, s, Me), 3.80 (s, CH3–O), 4.22 (1H, d, J = 7.65, N–CH2–S), 4.67 (1H, d, J = 7.65, N–CH2–S), 7.30–8.17 (14H, m, CH aromatic), 8.94 (1H, s, NH). 13C NMR (100 MHz, DMSO-d6): δC 21.62 (CH3-ph), 36.82 (C(CN)2), 57.35 (CH3 methoxy), 61.38 (N–CH2–S), 80.02 (C–Ar), 87.95 (C–O), 108.31, 117.56 (CN), 124.85, 126.25, 127.51, 128.23, 128.43, 128.66, 129.07, 132.83, 133.31, 134.01 (C aromatic), 136.97 (C–S), 137.02 (O–C–N). Anal. calcd for C28H22N4O4S (510.56): C, 65.87; H, 4.34; N, 10.97%. Found: C, 65.81; H, 4.37; N, 10.95%.Acknowledgements
The authors are gratefully acknowledge to the Research Council of Islamic Azad University, Shiraz branch for partial financial supporting of this work. The authors also thank Dr Hamid Reza Khavasi, faculty member of chemistry department of Shahid Beheshti University and his research group for his useful advice.References and notes
- (a) R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233 CrossRef CAS; (b) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS; (c) B. M. Trost, Science, 1991, 254, 1471 CAS.
- P. Tundo and P. T. Anastas, Green Chemistry: Challenging Perspectives, Oxford University Press, Oxford, 1999 Search PubMed.
- I. Akritopoulou-Zanze and S. W. Djuric, Top. Heterocycl. Chem., 2010, 25, 231 CAS.
- J. R. Sundberg, The Chemistry of Indoles, Academic, New York, 1996 Search PubMed.
- M. J. F. Da-Silva, J. S. Garden and C. A. Pinto, J. Braz. Chem. Soc., 2001, 12, 273 CrossRef.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- A. Lazuen Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846 RSC.
- S. Lindbæk Broman, M. Jevric, B. D. Andrew and M. Brøndsted Nielsen, J. Org. Chem., 2014, 79, 41 CrossRef PubMed.
- Y. A. Son, S. Y. Gwon, S. Y. Lee and S. H. Kim, Spectrochim. Acta, Part A, 2010, 75, 225 CrossRef PubMed.
- M. Fukuda, K. Kodama, H. Yamamoto and K. Mito, Dyes Pigm., 2004, 63, 115 CrossRef CAS.
- C. C. Lee and A. T. Hu, Dyes Pigm., 2003, 59, 63 CrossRef CAS.
- D. H. Lee, K. H. Lee and J. I. Hong, Org. Lett., 2001, 3, 5 CrossRef CAS PubMed.
- A. A. Ishchenko, A. V. Kulinich, S. L. Bondarev and V. N. Knyukshto, J. Phys. Chem. A, 2007, 111, 13629 CrossRef CAS PubMed.
- M. He, T. M. Leslie, J. A. Sinicropi, S. M. Garner and L. D. Reed, Chem. Mater., 2002, 14, 4669 CrossRef CAS.
- E. Knoevenagel, Chem. Ber., 1896, 29, 172 CrossRef CAS.
- K. Ebitani, Org. Synth., 2014, 2, 571 CAS.
- R. Y. Guo, Z. M. An, L. P. Mo, R. Z. Wang, H. X. Liu, S. X. Wang and Z. H. Zhang, ACS Comb. Sci., 2013, 15, 557 CrossRef CAS PubMed.
- T. Takahashi, Y. Li, F. Y. Tsai and K. Nakajima, Organometallics, 2001, 20, 595 CrossRef CAS.
- V. Nair, R. S. Menon, P. B. Beneesh, V. Sreekumar and S. Bindu, Org. Lett., 2004, 6, 767 CrossRef CAS PubMed.
- G. Qiu, Q. Ding, Y. Peng and J. Wu, Tetrahedron Lett., 2010, 51, 4391 CrossRef CAS.
- V. Nair, A. U. Vinod, N. Abhilash, R. S. Menon, V. Santhi, R. L. Varma, S. Viji, S. Mathew and R. Sriniva, Tetrahedron, 2003, 59, 10279 CrossRef CAS.
- H. R. Safaei, N. Shioukhi and M. Shekouhy, Monatsh. Chem., 2013, 144, 1855 CrossRef CAS.
- (a) H. R. Safaei, M. Shekouhy, A. Shirinfeshan and S. Rahmanpur, Mol. Diversity, 2012, 16, 669 CrossRef CAS PubMed; (b) H. R. Safaei, M. Shekouhy, S. Rahmanpur and A. Shirinfeshan, Green Chem., 2012, 14, 1696 RSC; (c) H. R. Safaei, M. Shekouhy, V. Shafiee and M. Davoodi, J. Mol. Liq., 2013, 180, 139 CrossRef CAS; (d) H. R. Safaei, M. Davoodi and M. Shekouhy, Synth. Commun., 2013, 43, 2178 CrossRef CAS; (e) H. R. Safaei, M. Shekouhy, S. Khademi, V. Rahmanian and M. Safaei, J. Ind. Eng. Chem., 2014, 20, 3019 CrossRef CAS; (f) J. A. Gladysz, H. R. Safaei and S. Nouri, Helv. Chim. Acta, 2014, 97, 1539 CrossRef CAS; (g) H. R. Safaei, M. Safaei and M. Shekouhy, RSC Adv., 2015, 5, 6797 RSC.
- H. A. Rouhi Sadabad, A. Bazgir, M. Eskandari and R. Ghahremanzadeh, Monatsh. Chem., 2014, 145, 1851 CrossRef.
- X. Wang, S.-Y. Wang and S.-J. Ji, Org. Lett., 2013, 15(8), 1954 CrossRef CAS PubMed.
- A. Sagırli, Y. Durust, B. Kariuki and D. W. Knight, Tetrahedron, 2013, 69, 69 CrossRef.
- X. Wang, X.-P. Xu, S.-Y. Wang, W. Zhou and S.-J. Ji, Org. Lett., 2013, 15(16), 4246 CrossRef CAS PubMed.
- M. B. Teimouri, A. Shaabanib and R. Bazhrang, Tetrahedron, 2006, 62, 1845 CrossRef CAS.
- F. Bigi, M. L. Conforti, R. Maggi, A. Piccinno and S. Giovanni, Green Chem., 2002, 2, 101 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22293a |
| This journal is © The Royal Society of Chemistry 2016 |





