A thermally remendable and reprocessable crosslinked methyl methacrylate polymer based on oxygen insensitive dynamic reversible C–ON bonds†
Abstract
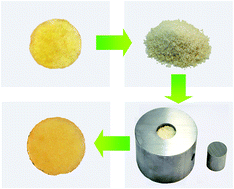
To improve the room temperature application stability of a dynamically reversible self-healing system based on alkoxyamine moieties, and to provide the polymer with oxygen insensitive self-healability, a crosslinked methyl methacrylate polymer with embedded alkoxyamine moieties that contain amido groups is prepared. Owing to synchronous fission/radical recombination of C–ON bonds, cracks in the polymer can be repeatedly healed without an additional catalyst, and the polymer itself can be remolded in the solid state or even decomposed by nitroxides in a controlled manner. The homolysis temperature of the dynamic reversible alkoxyamine derivative, which is closely related to crack healing and the reprocessing temperature of the polymer, is adjusted to be higher than room temperature by means of the appropriate electron-absorbing feature of amido groups. Meanwhile, the healing chemistry is coupled with air resistance. Besides, thiol–ene click chemistry is used to synthesize the target polymer. The possible side reaction that reduces the concentration of reversible bonds during polymerization is minimized accordingly.

 Please wait while we load your content...
Please wait while we load your content...