Production of biochars from Ca impregnated ramie biomass (Boehmeria nivea (L.) Gaud.) and their phosphate removal potential†
Abstract
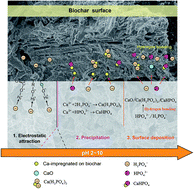
This work explored the efficiency and mechanisms of phosphate (P) removal by Ca-impregnated biochar prepared from CaCl2-pretreated ramie stem (Ca-RSB) and ramie bark (Ca-RBB). The properties of Ca-modified biochar were analyzed using elemental analysis, scanning electron microscopy (SEM), BET specific surface analysis, energy-dispersive X-ray analysis (EDS), Fourier transform infrared (FTIR) and a zeta potential meter. The results of characterization suggested that the Ca-RSB had a much higher H/C ratio, total pore volume, BET surface area and more functional groups compared with pristine biochar (RSB). In addition, a higher yield of Ca-RSB (50.8%) than RSB (28.0%) was also observed. Comparison experiments suggested that Ca-RSB showed higher adsorption capacity than Ca-RBB and the adsorption amount of Ca-RSB was more than two-folds that of RSB. Adsorption experimental data fitted well with pseudo-second order kinetics and the Langmuir isotherm. The intra-particle diffusion and Boyd's film-diffusion models revealed that the rate-controlled step was controlled by film-diffusion initially and then followed by intra-particle diffusion. Electrostatic attraction served as the main force to adsorb phosphates at a lower pH, and the precipitation and surface deposition took over at higher pH. The results of this study indicated that Ca-RSB is a potential effective and low-cost adsorbent for phosphate removal from wastewater.

 Please wait while we load your content...
Please wait while we load your content...