A novel chitosan/sponge chitin origin material as a membrane for supercapacitors – preparation and characterization
Izabela Stepniak*a,
Maciej Galinskia,
Krzysztof Nowackia,
Marcin Wysokowskib,
Paulina Jakubowskab,
Vasilii V. Bazhenovc,
Tilmann Leisegangc,
Hermann Ehrlichc and
Teofil Jesionowskib
aInstitute of Chemistry and Applied Electrochemistry, Poznan University of Technology, Berdychowo 4, 60965 Poznań, Poland. E-mail: izabela.stepniak@put.poznan.pl
bInstitute of Chemical Technology and Engineering, Poznan University of Technology, Berdychowo 4, 60965 Poznań, Poland
cInstitute of Experimental Physics, Technische Universität Bergakademie Freiberg, Leipziger Str. 23, 09596 Freiberg, Germany
First published on 23rd December 2015
Abstract
A new chitosan/sponge chitin – based membrane (CS/CH membrane) was prepared via the casting method for the first time. We used the demineralized skeleton of the marine demosponge Ianthella basta as a source for a chitinous network. The obtained membrane was immersed in 1 M LiOAc (lithium acetate) solution and tested in an Electric Double Layer Capacitor (EDLC) cell. For comparison, chitosan (CS) with LiOAc solution was also tested. The studies performed indicated good properties of the CS/CH membrane. Very good mechanical stability (for use in electrochemical capacitors) and electrochemical properties of the CS membrane were achieved by the addition of chitin isolated from the sponge to the polymer matrix. Their electrochemical performances were tested in EDLC cells by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge/discharge. The specific capacitances of the tested capacitor cells were found to be approximately 97 F g−1 and 88 F g−1 with CS/CH and CS membranes (in the voltage range 0–0.8 V), respectively.
Introduction
A huge increase in demand for a variety of portable devices, or hybrid and electric vehicles (HEVs, EVs) has been seen in recent years. This fact increases the importance and interest in energy storage devices. Electrochemical Double Layer Capacitors (EDLCs) are one of these electrochemical energy storage systems.1–3 Their use as advanced energy storage systems is constantly increasing.4,5 In comparison to most rechargeable battery systems, EDLCs have lower energy densities (4–8 W h kg−1), but much higher power densities (5–55 kW kg−1) and longer cycle life (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).6–8 They are currently widely investigated for many potential applications such as in back-up systems, hybrid vehicles, portable devices, and medical technology.9,10
000 cycles).6–8 They are currently widely investigated for many potential applications such as in back-up systems, hybrid vehicles, portable devices, and medical technology.9,10
EDLCs usually consist of two electrodes based on high surface area active materials (e.g. activate carbons),11 an electrolyte and a separator. Liquid electrolytes with a conductivity in the order of 10−2 S cm−1 are commonly used in a wide range of energy storage devices. However, their use in portable energy storage devices entails a risk of leakage. One of the methods for obtaining non-leaking energy storage devices is to place the liquid electrolyte in a polymeric matrix to form a hydrogel electrolyte (HGE), or a gel polymer electrolyte (GPE). Binding of liquid electrolyte in this manner also increases the safety of such devices. In this case, the gel electrolyte acts as a both separator and an ionic conductor between electrodes. The ionic conduction mechanism in this type of electrolyte should be very similar to that in liquid electrolyte, but gels have better shape flexibility compared to liquids.12
Consequently, in recent times, membranes/electrolytes are being intensively investigated for their use in electrochemical devices. Use of gel membranes and electrolytes prevents leakage of liquid electrolyte and facilitates preparation of more flexible, smaller and thinner electrochemical devices.
Currently, the majority of polymer matrices used are non-degradable. The use of environmentally friendly and widely available materials favors their development and reduces the impact of hazardous products on environment. Application of biodegradable materials in energy storage devices can contribute to the reduction of environmental damage. Chitosan is both environmentally friendly and a good membrane-forming polymer material, making it a good solution for many electrochemical applications.
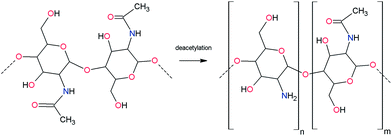
Chitosan is a linear polysaccharide consisting of β-(1-4)-linked-D-glucosamine and N-acetyl-D-glucosamine. This natural polymer is obtained by deacetylation of chitin (Scheme 1), which is commonly found in nature as the main structural component within the skeletal structures of fungi, diatoms, sponges, worms, molluscs, and mostly arthropods.13 In comparison to other polysaccharides, chitosan has several advantageous features; including biocompatibility, biodegradability, non-toxicity, the ability to produce thin films, excellent chemical-resistant and electrolytic properties, and ease of modification or cross-linking.
Application of chitosan-based membranes generally depends on both chemical and mechanical properties.14,15 Chitosan membranes are able to absorb and maintain a significant amount of solution (water or electrolyte). This property helps chitosan membranes achieve high ionic mobility (comparable to liquid electrolytes), which is important for the application of this type of electrolytes in energy storage devices. On the other hand, chitosan membranes have a low mechanical stability in swollen form. This fact limits their applications. In order to overcome these problems, chitosan is modified by different methods,16–19 such as blending, multilayer casting, or adding inorganic/organic reinforcements.
The thickness and strength of membranes applied in energy storage devices is another important aspect. Too great a swelling of the membrane in only one direction (increase in diameter) can cause it to weaken and consequently lead to a short circuit of electrodes in a device. In this study, we investigated the electrochemical properties of lithium acetic-doped chitosan/chitin membranes (CS/CH membrane) in EDLC cells.
For comparison, we tested the chitosan membrane (CS membrane) in 1 M lithium acetate aqueous solution. Chitin from the Ianthella basta sponge origin20 (Fig. 1) was used as reinforcement, holding the wet membrane dimensions. After membrane immersion in electrolyte, parallel chitosan chains move away from each other, which results in swelling of the membrane in one direction (a significant increase in thickness of the CS/CH membrane in comparison with CS membrane thickness).
 | ||
| Fig. 1 Skeletal fragment of Ianthella basta marine demosponge (A) was used for isolation of tube-like filigree chitinous scaffold (B). | ||
Experimental
Preparation of CH sponge
The isolation of the chitin-based scaffolds from the I. basta sponge that was collected as described previously20 were purchased by INTIB GmbH (Germany) and performed according to the following treatment steps:20Step 1: selected fragments of the sponge skeletons were cut into pieces of 10–30 cm2 and treated with 20% acetic acid at 37 °C for 12 h. Afterwards, the samples were rinsed three times with distilled water.
Step 2: the samples were treated with 2.5 M NaOH at 37 °C for 48 h. Afterwards, the samples were rinsed three times with distilled water.
Step 3: once more, the samples were treated with 2.5 M NaOH at 37 °C – now for 72 h. Afterwards, the specimens were rinsed three times with distilled water and stored under 4 °C. As reported previously,20 the crystal structure of the chitin-based scaffolds isolated accordingly was not influenced by the extraction procedure. A similar phenomenon is observed here (Fig. 1).
Preparation of CS solution
The 2 wt% solution of CS in 2 wt% acetic acid aqueous solution was obtained by dissolving an appropriate amount of CS powder (Sigma-Aldrich, DD = 75–85%) in acetic acid solution. The mixture was stirred at ambient temperature for 24 h. Finally, a pale yellow homogenous solution was obtained.Preparation of CS and CS/CH membranes
The CS and CS/CH membranes were prepared by a solution casting technique. The homogenous polymer solution was cast on a poly(propylene) plate with or without CH net (sponge CH) and left at 35 °C for 24 hours and then under vacuum at 25 °C (2 h) in order to evaporate the water. Subsequently, the membranes were immersed in 10 wt% sodium hydroxide aqueous solution for a few minutes at room temperature and then washed with de-ionized water and dried.Swelling of CS and CS/CH membranes in 1 M LiAc solution
The CS and CS/CH membranes were immersed in 1 M lithium acetate (LiOAc) solution to form electrolyte membranes. In order to determine the degree of swelling and the swelling ratio in the direction of length (diameter) and thickness of chitosan-based membranes the following procedure was performed. Membranes were dried in a vacuum, then 1 cm diameter (Dd – diameter for dry membrane) discs were cut out, weighed (Wd – dry weight) and their thickness (Td) was measured. Upon reaching equilibrium, the value of the wet weight (Ww), diameter (Dw) and thickness (Tw) were determined. The excess solution from the membrane surface was removed by draining it off with a piece of tissue paper. The degree of swelling (SD) and the swelling ratio in a direction of length (diameter) (DSL) and thickness (DST) were calculated as follows:| DS (%) = 100 × (Ww − Wd)/Wd | (1) |
| DSL (%) = 100 × (Dw − Dd)/Dd | (2) |
| DST (%) = 100 × (Tw − Td)/Td | (3) |
Assembly of EDLC cells
The symmetric EDLC cells were assembled from activated carbon cloths (Kynol® Europa GmbH, No ACC-507-20; specific surface area 2000 m2 g−1) used as the electrode materials with a CS membrane (ACC/CS/ACC) and CS/CH membrane (ACC/CS/CH/ACC). Before assembling the EDLC cells, all components (membranes and electrodes) were saturated with an aqueous lithium acetate solution (1 M LiOAc) and then placed in proper order (a sandwich system) into the test vessel. For two electrode and three-electrode measurements, Swagelock® systems were adapted. For a three electrode cell, a silver/silver chloride electrode was used as pseudo-reference electrode. Current collectors were made of gold. The final thickness of the assembled capacitors was approximately 1.2 mm.Electrochemical performance tests
The electrochemical performances of the capacitor cells were characterized using electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) and galvanostatic charge/discharge tests. EIS was carried out using an electrochemical system (μAutoLab FRA2 type III, EcoChemie, Netherlands) in a frequency range from 0.01 Hz to 99 kHz with a potential amplitude of 10 mV. Voltamperometric characteristics were determined with the same electrochemical system (μAutoLab FRA2 type III) in the voltage range 0–0.8 V with different scan rates 2–100 mV s−1. Galvanostatic charge/discharge tests were carried out using the Atlas 0461 MBI multichannel electrochemical system (Atlas-Sollich, Poland) at constant current values in the range of 5–50 mA, in the voltage range between 0 and 0.8 V. All tests were conducted in ambient conditions.The ionic conductivity of 1 M LiOAc was measured by the EIS method in the conductivity cell, with two parallel platinum electrodes at 25 °C.21
Tensile test
Mechanical properties of CS and CS/CH membranes were estimated by tensile test with universal testing machine Zwick Roell Z020 (Germany) with touchless extensometer video and a measurement head of 100 N. The examined samples were 10 × 30 mm in size. The stretching velocity was 10 mm min−1. Modulus of elasticity (Et), tensile strength (σM) and strain at break (εB) were determined.Results and discussion
The CS and CS/CH membranes were obtained by the casting methods. Fig. 2 presents CS and CS/CH-based obtained membranes with this procedure. Fig. 2A and B show pictures of CS/CH and CS based membranes. Chitin isolated from sponge is visible as a frame used in preparing the membrane for EDLC application.Fig. 2C and D present fluorescence microscopy and SEM images of the membranes showed in Fig. 2E and F. The properties of the membranes were characterized by uptake studies and electrochemical tests (ac impedance spectroscopy, cyclic voltammetry and galvanostatic charge/discharge methods) described in the following.
Electrolyte uptake studies
Dry CS and CS/CH membranes were filled with an aqueous solution of lithium acetate. The different behaviors of the membrane during that process were observed. A similar amount of electrolyte was absorbed during the swelling process of CS and CS/CH membranes, and a significant increase in the thickness of the filled membrane was observed for the CS/CH membrane. In the case of the CS membrane, a significant increase in the diameter was observed. The CH sponge served as a ‘frame’ or ‘form’, which kept the membrane dimensions in 2D and controlled its growth along the diameter. Therefore, the electrolyte absorbed has to find its place in the membrane, resulting in an increase in thickness. In the case of the membrane without the CH sponge, a significant increase in the diameter of the test membrane was observed. This led to a lower growth of thickness, with the same amount of electrolyte absorbed by the membrane. The increase in thickness of the membrane is supposed to be advantageous due to better penetration of the carbon fiber by a gel electrolyte and/or better contact between the electrolyte and the electrode material in the capacitor cell. To compare, the increase in diameter of membrane, at the cost of thickness, might weaken the contact between electrode material and electrolyte, which was confirmed by electrochemical tests (shown in electrochemical studies). This highlights the importance of controlling the swelling process of the membrane in both directions. The degree of swelling and swelling ratio in the direction of length (diameter) and thickness of CS and CS/CH membranes are shown in Table 1.| Membrane | Uptake (%) | SDD (%) | SDT (%) |
|---|---|---|---|
| CS | 205.2 | 33.0 | — |
| S/CH | 206.0 | 2.2 | 266.6 |
Cycling voltammetry studies
Fig. 3A shows the voltammetry curves obtained for all tested capacitor cells (ACC/CS/ACC and ACC/CS/CH/ACC). The voltammetric curves have the typical box-type shape pointing to an excellent charge propagation for each capacitor cell but lower capacitance is observed for the ACC/CS/ACC system. | ||
| Fig. 3 Cyclic voltammetry curves of EDLCs with CS (dotted line) and CS/CH (solid line) membrane, at a scan rate of 10 mV s−1 (A), and with CS/CH membrane at different scan rate (B). | ||
The specific capacitance values of capacitor cells were calculated from the corresponding CV curves using the equation: C = ∫Idt/(dE/dt)m−1, where I is the current, dE/dt is potential scan rate and m is mass of the electrode active material. The CS capacitor exhibits a minimum capacity equal to 75 F g−1 at a scan rate of 10 mV s−1 while capacity value of the CS/CH capacitor is 83 F g−1. This result demonstrates the excellent properties of the CS/CH membrane as a gel-electrolyte with comparable properties to liquid electrolytes. The weaker properties of the CS membrane as an electrolyte in the capacitor cell is related to the worse penetration of electrode material by the electrolyte. This may be associated with the decreased thickness of the CS membrane while swelling occurs in direction of the diameter. The decrease of charge propagation and the deviation from ideal box-like shape with the increase of scan rate from 2 to 100 mV s−1 are observed for all tested capacitor cells. Fig. 3B presents the CV curves of the CS/CH capacitor cell at different scan rates. This behavior is typical for double-layer capacitors. The CV measurements were also conducted in a three-electrode regime. The potential changes of positive and negative electrodes were measured separately in galvanostatic and potentiodynamic experiments.
Fig. 4 shows the results obtained for capacitor cells based on the CS/CH and the CS membranes. The shape of the box-like behavior suggests that the CS/CH based capacitor has the better charge distribution.
EIS studies
The Nyquist plots for all tested capacitor cells are presented in Fig. 5. In the high frequency range, a relatively large semicircle is visible only for the CS capacitor cell. The impedance spectrum of the CS/CH capacitor cell is shifted towards the lower impedance values Z. This is due to the better electrochemical characteristics of the CS/CH membrane (better penetration of the electrode material by gel electrolyte – see Electrolyte uptake studies) as compared to the CS membrane. In the low frequency region, the impedance spectrum for each capacitor cell is a straight line, which indicates a good capacitive behavior.6 | ||
| Fig. 5 AC impedance curves for the capacitors with CS and CS/CH membranes; 1 M LiOAc was used as an electrolyte. | ||
Fig. 6 present analysis of the capacitances obtained from EIS measurements. The frequency behavior modeling of a capacitor by using ac impedance data can be expressed as the relationships between real C′(ω) and imaginary C′′(ω) parts of capacitance with imaginary Z′(ω) and real Z(ω) impedance.22 C′(ω) corresponds to the capacitance of the cell that is measured during constant current charge/discharge (direct current), and C′′(ω) corresponds to energy dissipation. The typical frequency dependence of the real C′ and the imaginary C′′ capacitance as a function of the frequency (ω) for capacitor cells with CS/CH and CS membranes is presented in Fig. 6A and B. At a low frequency (1 mHz), the capacitance of the system reached 0.32 F and 0.29 F for the capacitor cell with the CS/CH and the CS membrane, respectively. This low frequency capacitance (Clf) corresponds to the cell capacitance measured during galvanostatic cycling at ±5 mA. When frequency is increased, capacitance decreases, and at a high frequency the capacitor behaves like a pure resistor.23 Fig. 6B shows the relationship between C′′ and the frequency for the tested devices. Maxima for CS/CH and CS capacitors were determined at 0.1034 Hz and 0.0648 Hz, respectively. From this graph, it is possible to deduce the relaxation time constant.24,25 This constant defines the border between capacitive behavior and resistive behavior of the capacitor.26 The relaxation time is inferred from frequency fo with to = 1/fo, where fo can be obtained from C′ = f(ω) plotted C′ = Clf/2 and from the C′′ = f(ω) plot (fo corresponds to the peak frequency). The relaxation time for the capacitor cell working with the CS/CH membrane is the shortest and is 9.7 s, whereas for the capacitor working with CS membrane is 15.4 s. The capacitor cell working with the CS/CH membrane is able to deliver its stored energy faster than the capacitor cell with the CS membranes, i.e. higher power can be obtained. The specific capacitance values for each cell were calculated with the equation C = 1/(2πfZ′), and shown as a function of frequency (ω = 2πf) in Fig. 6C. For the CS/CH capacitor cell, the capacitance is 83 F g−1 while for the CS capacitor cell, it is 74 F g−1.
Charge/discharge studies
Fig. 7 presents examples of galvanostatic charge/discharge curves of the CS/CH based capacitor. The typical triangular charge/discharge profile with a small potential drop for the each capacitor is observed. This type of capacitive behavior, together with the ideal box-like shaped CV curves, confirms good synergy between all materials in the cell.The capacitance of EDLC cells was calculated from the slope of the charge/discharge curve, which is C = I/(dU/dt)m−1, where C is the cell capacitance, I is the discharge current, dU/dt is slope of the discharge curve and m is the electrode mass.
From the galvanostatic charge/discharge curves, at the constant current of 5 mA, the specific capacitances of tested capacitor cells were found to be ca. 97 F g−1, 88 F g−1 with CS/CH and CS membranes, respectively. The same value of capacitance was obtained for the first and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000th cycle (Fig. 7A) for the CS/CH capacitor cell. The results indicate a good life cycle in this case. Table 2 presents the values of specific capacitance for all tested cells determined by galvanostatic charging and discharging.
000th cycle (Fig. 7A) for the CS/CH capacitor cell. The results indicate a good life cycle in this case. Table 2 presents the values of specific capacitance for all tested cells determined by galvanostatic charging and discharging.
| Capacitor | Ccharge/F g−1 | Cdischarge/F g−1 |
|---|---|---|
| CS/CH | 98 | 96 |
| CS | 89 | 87 |
Fig. 7B shows the behavior of the CS/CH-based EDLC in the three electrode measurements and the separately measured potential changes of positive and negative electrodes. The potential changes are in a similar order of magnitude, indicating similar capacitive properties of the carbon material on both sides of the capacitor cell.
The important electrical parameters of EDLCs are the energy and power density. The maximum energy density (Emax) may be estimated using the equation Emax = CU2/2, where C is the capacitance of two electrode capacitor cells and U is the operating voltage (the maximum cell voltage). The maximum power density (Pmax) may be calculated as Pmax = Emax/td, where td is the charge/discharge time for the voltage range used. The energy and power density have been calculated per unit mass of the electrode and are presented in Table 3.
| EDLC | Emax/W h kg−1 | Pmax/W kg−1 |
|---|---|---|
| CS/CH | 8.91 | 563 |
| CS | 7.91 | 554 |
Tensile test
Fig. 8 presents examples of stress–strain curves for CS and CS/CH membranes. Tensile properties are shown in Table 4. The obtained results clearly shows the improvement of mechanical properties by employing chitin sponge as the structural framework of membrane. Modulus of elasticity and tensile strength increased when adding chitin (approx. 5- and 4-fold respectively) comparing to the neat chitosan membrane. A slight decrease in strain at break is also noted. These results show that the CS/CH membrane is much stiffer and more durable material than CS membrane without chitinous scaffold of sponge origin.| Membrane | Et (MPa) | σM (MPa) | εB (%) |
|---|---|---|---|
| CS/CH | 5.25 ± 0.12 | 2.53 ± 0.10 | 40.85 ± 1.21 |
| CS | 0.98 ± 0.21 | 0.62 ± 0.17 | 48.24 ± 2.15 |
The elevated electrochemical characteristics obtained from potentiodynamic, galvanostatic and EIS measurements together with the mechanical improvement and electrolyte uptake, may be explained by the special tube-like and hierarchical morphology of the chitin-based scaffold. As reported previously, one of the advantages of sponge chitin in contrast to chitins of other origin is the ability to hold the electrolyte inside the tubular matrix.27
Conclusions
Chitin of sponge origin has broad application in modern disciplines like bioinspired materials science, tissue engineering and extreme biomimetics.27 A new chitosan/sponge chitin membrane (CS/CH membrane) was prepared by a casting method, and tested electrochemical performance in an EDLC cell, compared to the capacitor cells with chitosan membrane (CS membrane) containing the same electrolyte. The CS/CH membrane was found to possess better electrochemical properties compared to the CS membrane without chitinous scaffold of sponge origin. Better electrochemical properties and mechanical properties of the CS/CH membrane may arise from the specific structure of the chitinous scaffold.Acknowledgements
Authors are very thankful to Prof. Peter Schupp and to Prof. Dirk C. Meyer for great scientific discussion. Many thanks also to Prof. A. Ciszewski. This work was performed with the financial support from Ministry of Science and Higher Education Grants 03/31/DSPB/0297/2015 and 03/32/DSPB/0506/2015 – Poznan University of Technology; DFG Grant EH 394/3, BHMZ Programme of Erich-Krüger-Foundation (Germany) at TU Bergakademie Freiberg, and BMBF within the project CryPhysConcept 03 EK3029A.Notes and references
- A. D. Pasquier, I. Plitz, J. Gural, S. Menocal and G. Amatucci, J. Power Sources, 2003, 113, 62 CrossRef.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- K. S. Ryu, K. M. Kim, N. G. Park, Y. J. Park and S. H. Chang, J. Power Sources, 2002, 103, 305 CrossRef CAS.
- Supercapacitor, ed. F. Beguin and E. Frackowiak, Wiley-VCH, Weinheim, 2013 Search PubMed.
- W. Münchgesang, P. Meisner and G. Yushin, AIP Conf. Proc., 2014, 1597, 196 CrossRef.
- B. E. Conway, in Electrochemical Supercapacitors – Scientific Fundamentals and Technological Applications, Kluwer Academic/Plenum Publishers, New York, 1999 Search PubMed.
- O. Haas and E. J. Cairns, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 1995, 95, 163 RSC.
- R. Kotz and M. Carlen, Electrochim. Acta, 2000, 45, 2483 CrossRef CAS.
- I. Vroman and L. Tighzert, Materials, 2009, 2, 307 CrossRef CAS.
- A. Burke, J. Power Sources, 2000, 91, 37 CrossRef CAS.
- E. Frackowiak and F. Beguin, Carbon, 2001, 36, 937 CrossRef.
- M. Yang and J. Hou, Membranes, 2012, 2, 367 CrossRef CAS PubMed.
- H. Ehrlich, Int. Geol. Rev., 2010, 52, 661 CrossRef.
- H. Ehrlich, B. Krajewska, T. Hanke, R. Born, S. Heinemann, C. Knieb and H. Worch, J. Membr. Sci., 2006, 273, 124 CrossRef CAS.
- A. Anitha, S. Sowmya, P. T. Sudheesh Kumar, S. Deepthi, K. P. Chennazhi, H. Ehrlich, M. Tsurkan and R. Jayakumar, Prog. Polym. Sci., 2014, 39, 1644 CrossRef CAS.
- A. Chanachai, R. Jiraratananon, D. Uttapap, G. Y. Moon, W. A. Anderson and R. Y. M. Huang, J. Membr. Sci., 2000, 166, 271 CrossRef CAS.
- C. G. L. Khoo, S. Frantzich, A. Rosinski, M. Sjöström and J. Hoogstraate, Eur. J. Pharm. Biopharm., 2003, 55, 47 CrossRef CAS PubMed.
- L. Ma, C. Gao, Z. Mao, J. Zhou, J. Shen, X. Hu and C. Han, Biomaterials, 2003, 24, 4833 CrossRef CAS PubMed.
- Y. Huang, S. Onyeri, M. Siewe, A. Moshfeghian and S. V. Madihally, Biomaterials, 2005, 26, 7616 CrossRef CAS PubMed.
- E. Brunner, H. Ehrlich, P. Schupp, R. Hedrich, S. Hunold, M. Kammer, S. Machill, S. Paasch, V. V. Bazhenov, D. V. Kurek, T. Arnold, S. Brockmann, M. Ruhnow and R. Born, J. Struct. Biol., 2009, 168, 539 CrossRef CAS PubMed.
- I. Stepniak, E. Andrzejewska, A. Dembna and M. Galinski, Electrochim. Acta, 2014, 121, 27 CrossRef CAS.
- Impedance Spectroscopy, ed. E. Barsoukov and J. R. Macdonald, John Wiley & Sons, Inc., New Jersey, 2005 Search PubMed.
- R. de Levis, Electrochim. Acta, 1963, 8, 751 CrossRef.
- M. A. Ratner and D. F. Shriver, Chem. Rev., 1988, 88, 109 CrossRef CAS.
- P. L. Taberna, P. Simon and J. F. Fauvarque, J. Electrochem. Soc., 2003, 150, A292 CrossRef CAS.
- J. H. Jang, A. Kato, K. Machida and K. Naoi, J. Electrochem. Soc., 2006, 153, A321 CrossRef CAS.
- M. Wysokowski, I. Petrenko, A. L. Stelling, D. Stawski, T. Jesionowski and H. Ehrlich, Polymers, 2015, 7, 235 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |