Iron-containing platinum-based catalysts as cathode and anode materials for low-temperature acidic fuel cells: a review
Ermete Antolini
Scuola di Scienza dei Materiali, Via 25 aprile 22, 16016 Cogoleto, Genova, Italy. E-mail: ermantol@libero.it
First published on 17th December 2015
Abstract
The high availability and low cost of Fe make it an interesting element for use in non-precious Pt-free catalysts and Pt-based catalysts for low-temperature fuel cells. Pt–Fe compounds can present three crystal structures, these are a disordered fcc PtxFe alloy and two ordered intermetallic alloys (fcc Pt3Fe and fct PtFe types). Fe-containing Pt-based binary and ternary catalysts in the different crystal structures have been tested both as anode and cathode materials in low-temperature acid fuel cells. In this work an overview of the application of Fe-containing catalysts as cathode materials for oxygen reduction and as anode materials for methanol and ethanol oxidation in low-temperature polymer electrolyte fuel cells fuelled with hydrogen or low molecular weight alcohols, is presented. Moreover, the stability of iron in Pt-based binary and ternary catalysts towards dissolution in acid medium is discussed.
1. Introduction
Low-temperature polymer electrolyte membrane fuel cells fuelled with hydrogen (PEMFCs) or low molecular weight alcohols such as methanol (direct methanol fuel cells, DMFCs) and ethanol (direct ethanol fuel cells, DEFCs) represent an environmentally friendly technology and arouse considerable interest as a means to generate electricity by direct electrochemical conversion of hydrogen/alcohol and oxygen into water/water, carbon dioxide and/or partial alcohol oxidation products.1–3 Platinum has the highest catalytic activity for oxygen reduction of any of the pure metals and for alcohol oxidation at low temperature (<100 °C) in an acidic environment of any of the pure metals, thus unsupported and carbon supported platinum nanoparticles are commonly used as fuel cell electrocatalysts. The high cost of platinum and kinetic limitations of the oxygen reduction reaction (ORR), when Pt is used as a cathode catalyst, and the poisoning of platinum by strongly adsorbed species coming from the dissociative adsorption of alcohol, when Pt is used as an anode catalyst, represent, however, a serious obstacle to the marketing of low-temperature fuel cells. Thus, research efforts to obtain ORR catalysts more active, more alcohol-resistant and less expensive than Pt have been addressed to binary and ternary Pt-based4,5 and Pt-free catalysts.6,7 In the same way, to reduce both the poisoning of Pt and the cost of the anode, Pt-based8,9 and non-Pt catalysts have been extensively investigated.10,11Iron (Fe) is a metal in the first transition series. It is by mass the most common element on Earth, forming much of Earth's outer and inner core. The high availability and low cost of Fe make it an interesting element for its use in non-precious Pt-free catalyst and in Pt-based catalysts for low-temperature fuel cells. In this work an overview of the use of iron in Pt-based catalysts as cathode materials for the ORR, and as anode materials for the methanol oxidation reaction (MOR) in DMFCs and the ethanol oxidation reaction (EOR) in DEFCs, is presented. As a key issue, the stability of Fe containing Pt-based catalysts in fuel cell environment is widely discussed.
2. Structural characteristics of Pt–Fe alloys
Pt–Fe compounds present three crystal structures, that is, a disordered PtxFe alloy and two ordered intermetallic alloys (Pt3Fe and PtFe types). The chemically disordered PtxFe alloy has a face-centered cubic (fcc) structure (A1 phase, space group: Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) in which the Pt and Fe atoms are randomly distributed in all (0, 0, 0), (1/2, 1/2, 0), (1/2, 0, 1/2) and (0, 1/2, 1/2) crystallographic sites.12,13 By annealing, the disordered fcc Pt3Fe structure can be converted to chemically ordered fcc Pt3Fe structure (L12 phase, space group: Pm
m) in which the Pt and Fe atoms are randomly distributed in all (0, 0, 0), (1/2, 1/2, 0), (1/2, 0, 1/2) and (0, 1/2, 1/2) crystallographic sites.12,13 By annealing, the disordered fcc Pt3Fe structure can be converted to chemically ordered fcc Pt3Fe structure (L12 phase, space group: Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).12 In the ordered fcc Pt3Fe structure Fe atoms occupy the eight corner positions and Pt atoms occupy six-face centered positions in the cube. Unlike the fcc structure, in the ordered face-centered tetragonal (fct) PtFe structure (L10 phase, space group: P4/mmm) the Fe and Pt atoms form alternating layers within the crystal lattice, where Pt is in (0, 0, 0) and (1/2, 1/2, 0) sites, and Fe is in (1/2, 0, 1/2) and (0, 1/2, 1/2) sites.13,14 The fct structure breaks the cubic symmetry of the fcc structure by altering the length of one of the sides. The ordered fct PtFe structure can be obtained by annealing of the disordered fcc structure. However, to avoid particle aggregation, the ordered phase can be directly prepared by deposition onto heated substrates15 or by physical deposition processes.14 The crystal structures of the disordered PtxFe phase and the two ordered phases are shown in Fig. 1.
m).12 In the ordered fcc Pt3Fe structure Fe atoms occupy the eight corner positions and Pt atoms occupy six-face centered positions in the cube. Unlike the fcc structure, in the ordered face-centered tetragonal (fct) PtFe structure (L10 phase, space group: P4/mmm) the Fe and Pt atoms form alternating layers within the crystal lattice, where Pt is in (0, 0, 0) and (1/2, 1/2, 0) sites, and Fe is in (1/2, 0, 1/2) and (0, 1/2, 1/2) sites.13,14 The fct structure breaks the cubic symmetry of the fcc structure by altering the length of one of the sides. The ordered fct PtFe structure can be obtained by annealing of the disordered fcc structure. However, to avoid particle aggregation, the ordered phase can be directly prepared by deposition onto heated substrates15 or by physical deposition processes.14 The crystal structures of the disordered PtxFe phase and the two ordered phases are shown in Fig. 1.
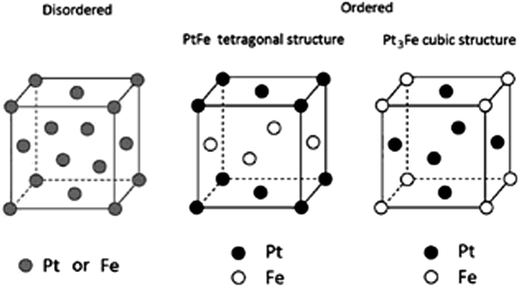 | ||
Fig. 1 Crystal structure of disordered PtxFe alloys [Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m], ordered tetragonal PtFe [P4/mmm] and ordered cubic Pt3Fe [Pm m], ordered tetragonal PtFe [P4/mmm] and ordered cubic Pt3Fe [Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m]. Reproduced from ref. 13, copyright 2012, with permission from Elsevier. m]. Reproduced from ref. 13, copyright 2012, with permission from Elsevier. | ||
During the synthesis of bimetallic nanoparticles (NPs), a compositional difference between the bulk and surface may be induced by differences in the surface energies of the alloying elements and changes in the interaction energies between them. This compositional difference, named surface segregation, influences the catalytic activities of alloys.16 Regarding surface segregation in PtFe alloys, first Burton and Polizzotti,17 using Auger electron spectroscopy, found no evidence of any surface segregation in a 2 at% solid solution of Fe in Pt. Surface segregation in both fcc and fct PtFe alloys has been the subject of theoretical studies. Spencer18 proposed a broken-bond model with thermodynamic data for fcc PtFe alloys and for pure metals to predict surface segregation. Based on this model, little segregation of either iron or platinum would be expected in the range 2–20 at% Fe. Fct PtFe nanoparticles show reduced L10 order with decreasing particle size. The phenomenon was addressed by investigating the thermodynamic driving forces for surface segregation using a local (inhomogeneous) cluster expansion fit to ab initio data.19 Subsequent Monte Carlo simulations reveal that first surface layer Pt segregation is compensated by Pt depletion in the second subsurface layer. This indicates that the core ordered state is not affected by surface thermodynamics. Yamakawa et al.20 carried out phase-field simulations to investigate phase transformation and surface segregation in Pt-based alloy nanoparticles. The alloy element with the lower surface energy had a tendency to segregate near the surface. However, the attractive interaction energy between platinum and the alloyed transition metal may prevent the alloy from decomposing. In the case of fct FePt, the strong attractive interaction energy between Fe and Pt overcame the surface energy differences. As can be seen in Fig. 2, where the degree of surface segregation in the alloy particles is expressed by the numerical values in parentheses, no segregation occurs in the FePt particle, despite the surface energy difference, because of the effect of large mixing energy.
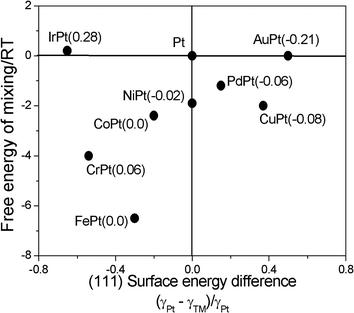 | ||
| Fig. 2 Trend of surface atomic segregation of Pt-based binary nanoparticles. The number within the parentheses following the name of each alloy element is the value calculated using the equation (∑cPt − ∑cTM)/∑(cPt + cTM) (cPt and cTM are the local atomic concentration of Pt and transition metal) for the region where r ≥ (d/2 − 0.5) (d is the particle diameter in nm), which corresponds to the surface shell of the nanoparticle. Numerical calculation results were obtained for a particle with a diameter of 3 nm at 700 °C. Reproduced from ref. 20, copyright 2014, with permission from Elsevier. | ||
3. Oxygen reduction on Pt–Fe catalysts
3.1 General overview
The ORR is a complex reaction, going through different steps and reaction intermediates.21 Damjanovic and Brusic22 proposed that the electroreduction of oxygen on Pt in acid media takes place though both a mechanism with oxygen electrochemisorption:| O2 + Pt + H+ + e− ⇌ Pt–O2Hads | (1) |
| Pt–O2Hads + 3H+ + 3e− ⇌ Pt + 2H2O | (2) |
| O2 + Pt + H+ + e− ⇌ Pt–O2Hads | (3) |
| Pt–O2Hads + H2O ⇌ 3Pt–OHads | (4) |
| 3Pt–OHads + 3H+ + 3e− ⇌ 3H2O | (5) |
The reaction is first order with respect to O2 pressure in the whole range of potentials. The high cost and the limited resources of platinum, as well as the low rate of the cathode reaction, prompted the development of binary and ternary Pt-based alloys23–28 and Pt-free catalysts.28–34 Pt alloyed with first row transition metals presented a significant improvement of the ORR activity with respect to Pt alone by a direct four-electron reaction without involving the intermediate hydrogen peroxide step. Norskov et al.,35 using density functional theory calculations, developed a model providing a volcano-type relationship between the rate of the cathode reaction and the oxygen adsorption energy. Their model showed that Pt is the best elemental cathode material and that alloying improves its performance. The enhancement of the ORR activity on Pt alloys was attributed to geometric factors (decrease of the Pt–Pt bond distance),36 dissolution of the more oxidisable alloying component,37 change in surface structure38 or electronic factors (increase of Pt d-electron vacancy), associated to the formation of a Pt skin on the catalyst surface.39,40 Mukerjee et al.41 ascribed the increase of the ORR activity to the interplay between the electronic and geometric factors and their effect on the chemisorption behaviour of OH species from the electrolyte. Recently, Jia et al.42 developed a modeling approach based on FEFF8 (FEFF8 is an “ab initio” real space multiple-scattering (RSMS) code for simultaneous calculation of X-ray absorption spectroscopy (XAS) spectra and electronic structure) calculations to study the relationships between the atomic structure, electronic property, and ORR activity of Pt3M nanoparticles (NPs) in combination with experimental results. They developed a representative cluster model of Pt3M (M = Cr, Mn, Fe, Co, Ni) NPs, namely Pt19M6, and showed that the calculated Pt surface d-band center εd can be directly related to the ORR activity enhancement trends of Pt3M NPs in cathode catalysts (lower εd corresponding to higher activity), as shown in Fig. 3.
 | ||
| Fig. 3 Variations in the d-band center for Pt19M6 clusters and experimental ORR specific activities measured at 0.9 V in a proton exchange membrane fuel cell for nanoscaled Pt3M/C alloys (green). Reproduced from ref. 42, copyright 2013, with permission from Elsevier. | ||
3.2 Oxygen reduction on fcc Pt–Fe catalysts
The ORR specific activity (SA, activity (active area)−1) of fcc Pt–Fe alloys has been extensively investigated.13,41–65 Iron itself is not an active site for ORR, but alloying it with Pt, in agreement with the considerations reported in the previous paragraph, results in an enhancement of the ORR activity of platinum, due both geometric and electronic effects. In the former case, the formation of fcc Pt–Fe alloys gives rise to the shrinkage of the metal lattice parameter results in a more favourable Pt–Pt distance for weakening Pt–oxygen bond and facilitating the dissociative adsorption of O2. In the latter case, at Pt–Fe bulk alloys it was observed that the Pt–Fe alloy catalyst surface, consisting of a Pt skin of a few monolayers, is modified in the electronic structure by that of the bulk alloy.43 By the addition of Fe up to 50 at%, the 5d vacancies of the surface increase. Such an increase of 5d vacancies led to an increase 2π electron donation from O2 to the surface Pt, resulting in an increased O2 adsorption and a weakening of the O–O bond. As a results, scission of the bond must occur instantaneously as electrons are back donated from 5d orbitals of the surface Pt to 2π* orbitals of the adsorbed O2. When the 5d vacancy of the electrode increases more or the Fermi level further lowers by addition of the second element beyond each optimum content, the Pt–O bonding becomes stronger and the back donation becomes difficult, resulting in the lowered O2 reaction rate. Moreover, a chemical effect was also observed: non-alloyed Fe is more prone to corrode at the fuel cell operation voltage at 90 °C, and the Fe ions could promote the ORR activity.44 Li et al.44 found that the limited currents for Pt/C catalyst increased considerably when 100 or 1000 ppm Fe ion was added in HClO4 electrolyte, in agreement with the result of Sun and Tseung,66 which observed that the ORR activity of Pt/C in a H2SO4 solution in the presence of 100 ppm Fe is doubled at 70 °C and 0.6 V vs. SHE. The mechanism of enhancement was ascribed to the reaction of dissolved Fe and H2O2 to form Fe3*hydroperoxy complexes, followed by H2O2 decomposition to H2O. The dependence of the Pt–Fe to Pt ORR specific activity ratio (SAPt–Fe/SAPt) in acid media on Fe content in the catalyst by different datasets44–49 is shown in Fig. 4A. Generally, a positive effect of Fe addition to Pt on the ORR activity can be observed. However, as can be seen in Fig. 4A, the data are scattered, generally each database presenting a maximum of (SAPt–Fe/SAPt) at different values of Fe content, likely depending on the preparation method, and hence on alloying degree and particle size. As shown in Fig. 4B, where PtFe fcc lattice parameters from different datasets44,46,48 are plotted against iron content in the catalyst, almost all the experimental values of the lattice parameters are above the line obtained by assuming that the relationship between fcc lattice parameter and Pt1–xFex alloy composition obeys to the Vegard's law for x in the range 0–0.5, and using the lattice parameter values 0.392 nm (ref. 67) and 0.384 nm (ref. 68 and 69) for pure Pt and Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively, indicating that the amount of Fe alloyed is lower than the overall Fe content in the catalyst and depends on database. The dependence of (SAPt–Fe/SAPt) on fcc lattice parameter from different datasets13,44,46,48,65 is shown in Fig. 4C: the curve goes through a maximum at 0.3896 nm, corresponding to an iron content in the alloy of 14 at%. As can be seen in Fig. 4C, the value of (SAPt–Fe/SAPt) for the ordered fcc Pt3Fe alloy13,65 is higher than the value of the disordered fcc Pt3Fe alloy, according to the curve resulting from the (SAPt–Fe/SAPt) values of disordered alloys. The effect of ordering in fcc Pt3Fe alloys on the ORR activity was evaluated.13,56 Chen et al.13 observed that the SA of fully ordered Pt3Fe/C is higher than that of a slightly disordered Pt3Fe/C alloy. Conversely, Hwang and Chung56 found that the specific activity of Pt3Fe/C alloys is independent of the ordering degree.
1), respectively, indicating that the amount of Fe alloyed is lower than the overall Fe content in the catalyst and depends on database. The dependence of (SAPt–Fe/SAPt) on fcc lattice parameter from different datasets13,44,46,48,65 is shown in Fig. 4C: the curve goes through a maximum at 0.3896 nm, corresponding to an iron content in the alloy of 14 at%. As can be seen in Fig. 4C, the value of (SAPt–Fe/SAPt) for the ordered fcc Pt3Fe alloy13,65 is higher than the value of the disordered fcc Pt3Fe alloy, according to the curve resulting from the (SAPt–Fe/SAPt) values of disordered alloys. The effect of ordering in fcc Pt3Fe alloys on the ORR activity was evaluated.13,56 Chen et al.13 observed that the SA of fully ordered Pt3Fe/C is higher than that of a slightly disordered Pt3Fe/C alloy. Conversely, Hwang and Chung56 found that the specific activity of Pt3Fe/C alloys is independent of the ordering degree.
A way to improve the activity and stability of Pt–Fe/C catalysts is their synthesis in a nanostructured form with high surface area. PtxFe-nanowires50 and Pt–Fe flower-like nanoclusters51 showed much higher ORR activity and stability than Pt/C. Three different types of alloyed Pt–Fe nanostructures, nanodendrites, nanospheres and nanocubes, were prepared and their ORR activities were investigated.52 The ORR catalytic activity of the nanostructures increased in the order Pt/C < Pt–Fe nanospheres < Pt–Fe nanocubes < Pt–Fe nanodendrites. The enhancement could be attributed to the exposure of high-index {311} facet of the nanodendrite with high surface energy in comparison to that low-index {111} and {200} facets of nanospheres and nanocubes, respectively. Core/shell Pt–Fe@Pd catalysts with a 5 nm Pd core and a FePt shell whose thickness is tunable from 1 to 3 nm were synthesized by Mazumder et al.53 The ORR activity of the Pt–Fe@Pd catalysts was dependent on the Pt–Fe shell thickness. At the half-wave potential, the current density from the 5 nm/1 nm Pt–Fe@Pd catalyst was ca. 12 and 15 times higher than those from a commercial Pt and the 5 nm/3 nm Pt–Fe@Pd catalyst.
3.3 Oxygen reduction on fcc Pt–Fe catalysts in the presence of methanol
The crossover of methanol from the anode to the cathode side through the proton-exchange membrane represents a considerable drawback to the DMFC development.70 The methanol oxidation reaction needs multiple sites for the adsorption of methanol and sites containing oxygenated species for the oxidation of adsorbed methanol residues. A direct reaction between methanol and oxygen can occur owing to the adsorption of methanol on cathode Pt sites, reducing the cell voltage, generating additional water and increasing the required oxygen stoichiometric ratio. Moreover, the methanol oxidation intermediate species, such as CO, can poison the Pt surface, resulting in a reduction of cell performance. Thus, catalysts with lower activity for methanol oxidation than Pt to decrease the mixed potential and, on the other hand, catalysts with higher MOR activity to decrease CO poisoning have been investigated. The so-called “ensemble effect” is a way to decrease methanol adsorption. The ensemble effect, consist in the dilution of the active component with non catalytically active metals by alloying.71 The dilution effect due to presence of non-active atoms around Pt active sites could reduce methanol adsorption on Pt sites.72 Conversely, oxygen adsorption, requiring only two adjacent sites, is not influenced by the presence of the second metal. Moreover, geometric and electronic effects by alloying can decrease methanol adsorption. The ORR activity of some Pt–M alloys (M = first-row transition metals), having a higher ORR activity for the ORR than Pt in the absence of methanol, was evaluated in the presence of methanol.58 Among them, the ORR activity of fcc Pt–Fe/C catalysts in the presence of methanol has been investigated and, generally, a higher methanol tolerance of these catalysts than that of Pt/C has been reported.57–64 Fig. 5A and B, obtained from data in ref. 57 and 58, shows the dependence of the methanol oxidation effect coefficient ((jORR − jORR/methanol)/jORR) of Pt–Fe/C and Pt/C catalysts on methanol concentration. As can be seen in Fig. 5, independently of methanol concentration, the methanol oxidation effect coefficient of all Pt–Fe catalysts is considerably lower than that of Pt/C and slightly decreases with decreasing PtFe alloy lattice parameter. For low Fe contents the higher methanol tolerance has to be ascribed to the ensemble effect, while for high Fe content the enhanced methanol tolerance is also due to the modified electron structure of Pt atoms by Fe atoms (downshift of the Pt d-band center), leading to a decreased Pt poisoning by methanol oxidation intermediate species. According to Gong et al.60 and Malheiro et al.,61 instead, the Pt–Fe/C catalysts with Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe atomic compositions 9
Fe atomic compositions 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed enhanced methanol tolerance and catalytic activity than the catalyst with Pt
1 showed enhanced methanol tolerance and catalytic activity than the catalyst with Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe 3
Fe 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As the values of the lattice parameter, and hence the amount of Fe alloyed, of Pt–Fe/C catalysts with Pt/Fe atomic composition 2.3
1. As the values of the lattice parameter, and hence the amount of Fe alloyed, of Pt–Fe/C catalysts with Pt/Fe atomic composition 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5
1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are very close, the highest methanol tolerance of the catalyst with Pt/Fe atomic composition 1
1 are very close, the highest methanol tolerance of the catalyst with Pt/Fe atomic composition 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has to be ascribed to the presence of a high amount of non-alloyed Fe, likely in the oxide form, supporting the full oxidation of adsorbed methanol oxidation intermediate species.61 Finally, two works reported no higher methanol tolerance of Pt–Fe/C than Pt/C, and the higher activity of the binary catalysts than Pt was ascribed only to the higher activity for oxygen reduction of the binary catalysts.44,73
1 has to be ascribed to the presence of a high amount of non-alloyed Fe, likely in the oxide form, supporting the full oxidation of adsorbed methanol oxidation intermediate species.61 Finally, two works reported no higher methanol tolerance of Pt–Fe/C than Pt/C, and the higher activity of the binary catalysts than Pt was ascribed only to the higher activity for oxygen reduction of the binary catalysts.44,73
 | ||
| Fig. 5 Dependence of the methanol oxidation effect coefficient ((jORR − jORR/methanol)/jORR) of Pt–Fe/C and Pt/C catalysts on methanol concentration from data in ref. 57 (A) and ref. 58 (B). | ||
3.4 Stability of fcc Pt–Fe catalysts
The effect of the degree of alloying of carbon supported Pt–Fe (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with similar particle size on sintering resistance and Pt corrosion resistance was investigated.74 An accelerated ageing test simulating phosphoric acid fuel cell (PAFC) conditions was carried out on Pt–Fe/C catalysts with different alloying degree, by immersion of the catalysts on H3PO4 at 204 °C for 5 h in air under open circuit conditions. As can be seen in Fig. 6, both Pt corrosion resistance and, in particular, sintering resistance increased with increasing the alloying degree. The high Pt corrosion resistance of fully alloyed Pt3Fe was explained by Greeley and Nørskov by first-principles calculations.75 They found that the dissolution potential of Pt in Pt “skin” layers from Pt3M (M = Fe, Co, and Ni) bulk alloys increased by 0.19, 0.16, and 0.14 V for Pt3Fe, Pt3Co, and Pt3Ni, respectively, with respect to the dissolution potential of pure Pt.
1) with similar particle size on sintering resistance and Pt corrosion resistance was investigated.74 An accelerated ageing test simulating phosphoric acid fuel cell (PAFC) conditions was carried out on Pt–Fe/C catalysts with different alloying degree, by immersion of the catalysts on H3PO4 at 204 °C for 5 h in air under open circuit conditions. As can be seen in Fig. 6, both Pt corrosion resistance and, in particular, sintering resistance increased with increasing the alloying degree. The high Pt corrosion resistance of fully alloyed Pt3Fe was explained by Greeley and Nørskov by first-principles calculations.75 They found that the dissolution potential of Pt in Pt “skin” layers from Pt3M (M = Fe, Co, and Ni) bulk alloys increased by 0.19, 0.16, and 0.14 V for Pt3Fe, Pt3Co, and Pt3Ni, respectively, with respect to the dissolution potential of pure Pt.
The dissolution of Fe from fcc Pt1–xFex (0 < x < 1) catalysts under PEMFC conditions was investigated.76 Electron microprobe measurements showed that iron is removed from all compositions during acid treatment at 80 °C, but that the percentage removed increases with x, acid strength, and temperature. For values of x < 0.6 no substantial changes in the lattice size are observed upon dissolution of Fe, suggesting that the dissolved iron originates from the surface. However, for values of x > 0.6, the increase of lattice parameter indicated that Fe dissolves also from the bulk. X-ray photoelectron spectroscopy (XPS) measurements showed complete removal of surface Fe after acid treatment at 80 °C for all compositions.
Colón-Mercado and Popov77 developed an accelerated durability test (ADT) to evaluate the long-term performance of the Pt–M/C (M = Fe, Co, Ni) catalysts. The ADT cell consists of a three-electrode system, including a reference electrode, a platinum mesh counter electrode and the catalyst-coated gas diffusion layer as a working electrode. The electrodes were immersed in a H2SO4 solution, which mimics the environment of the electrode–membrane interface on PEMFC cathode side. Unlike the case of an electrode–membrane assembly (MEA) interface, in which only the catalyst in contact with the membrane is active, in the case of the ADT the entire active surface area of the catalyst is exposed to proton, since the electrode is completely immersed in the electrolyte. Under this specific condition, the deterioration of the catalysts is accelerated. The non-noble metal dissolution rate in a H2SO4 solution was evaluated for different Pt-alloy catalysts at 0.8 V vs. NHE. The highest metal loss was observed for the samples with a Pt to non-noble metal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After 50 h ADT, ca. 40% Fe dissolved from fcc Pt–Fe/C (1
1. After 50 h ADT, ca. 40% Fe dissolved from fcc Pt–Fe/C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), showing a high nearly constant dissolution rate and no dissolution suppression up to 70 h. The ORR current starts to decrease rapidly for the first 100 h until it reaches a slow, but steady decay. Among the Pt–M/C catalysts, the highest initial current was observed for Pt–Fe/C. However, its activity decay was the highest when compared to the other alloy catalysts.
1), showing a high nearly constant dissolution rate and no dissolution suppression up to 70 h. The ORR current starts to decrease rapidly for the first 100 h until it reaches a slow, but steady decay. Among the Pt–M/C catalysts, the highest initial current was observed for Pt–Fe/C. However, its activity decay was the highest when compared to the other alloy catalysts.
Repetitive potential cycling (RPC) is a widely used technique to evaluate the stability of a catalyst. Generally, the ageing test by repetitive potential cycling is more severe than under steady-state conditions.78 The growth of metal particles of supported catalysts by repetitive potential cycling has been reported. In the case of platinum alloys, both the non-noble metal particles and Pt dissolve into the electrolyte. Then the dissolved Pt redeposits on the surface of larger particles (Ostwald ripening), resulting in a Pt surface-enrichment.79 The stability of fcc Pt–Fe catalysts was evaluated by potential cycling tests in different works.47,48,50,51,80 RPC affects the electrochemically active surface area (ECSA) and the ORR activity of Pt–Fe and Pt catalysts. Generally, while for Pt catalysts only a monotonous decrease of the ECSA occurs, due to Pt particle growth, potential cycling of binary Pt–Fe catalysts gives rise to an increase of the ECSA within the first cycles,80–83 associated to Fe dissolution, then, to a decrease in the active area, due to the growth of the particles. The reason of ECSA increase is that leaching of iron near the surface exposed further Pt or caused surface roughening. Regarding the ECSA increase, Zhang et al.50 observed a remarkable increase in ECSA of Pt–Fe/C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) within the first 150 cycles, a small ECSA increase at around 50 cycles for the Pt–Fe/C (2
1) within the first 150 cycles, a small ECSA increase at around 50 cycles for the Pt–Fe/C (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and no increase for the Pt–Fe/C (3
1), and no increase for the Pt–Fe/C (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A similar trend was observed by Malheiro et al.,80 thus highlighting the dependence of the increase of the active area on iron content. Following the reaching of the peak, for the Pt1–xFex catalysts with x < 0.5 the ECSA of Pt–Fe/C catalysts decreased more slowly than pure Pt with cycling, reflecting the rearrangement of the remaining Pt layer and the formation of a Pt skin layer, which protects the underlying bulk alloy from corrosion.75,81 On the other hand, the Pt–Fe/C catalyst with 50 at% Fe showed a similar50 or higher49 decrease of the ECSA than Pt/C, indicating no formation of a stable Pt skin. Generally, both unsupported47,50 and carbon supported49,51 Pt–Fe catalysts showed a higher stability of the ORR activity than Pt/C. The higher stability of the ORR activity of Pt1–xFex catalysts with x < 0.5 after RPC indicated that, in addition to a higher ECSA stability, Fe loss do not appreciably affects the specific activity of these catalysts.
1). A similar trend was observed by Malheiro et al.,80 thus highlighting the dependence of the increase of the active area on iron content. Following the reaching of the peak, for the Pt1–xFex catalysts with x < 0.5 the ECSA of Pt–Fe/C catalysts decreased more slowly than pure Pt with cycling, reflecting the rearrangement of the remaining Pt layer and the formation of a Pt skin layer, which protects the underlying bulk alloy from corrosion.75,81 On the other hand, the Pt–Fe/C catalyst with 50 at% Fe showed a similar50 or higher49 decrease of the ECSA than Pt/C, indicating no formation of a stable Pt skin. Generally, both unsupported47,50 and carbon supported49,51 Pt–Fe catalysts showed a higher stability of the ORR activity than Pt/C. The higher stability of the ORR activity of Pt1–xFex catalysts with x < 0.5 after RPC indicated that, in addition to a higher ECSA stability, Fe loss do not appreciably affects the specific activity of these catalysts.
3.5 Stability and ORR activity of fct Pt–Fe catalysts
Unlike fcc disordered Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalysts, ordered fct Pt–Fe catalysts presented low Fe dissolution in acid solution under steady-state conditions,82–85 however, as in the case of fcc Pt–Fe (1
1) catalysts, ordered fct Pt–Fe catalysts presented low Fe dissolution in acid solution under steady-state conditions,82–85 however, as in the case of fcc Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), they are not stable under potential cycling.84,85 Firstly, Shukla et al.82 suspended a fct Pt–Fe/C catalyst in a H2SO4 solution at 85 °C for 16 h. Little difference in the XRD patterns of this catalyst before and after test in H2SO4 was observed. They found that only 0.6 ppm of Fe leached from the alloy sample during the first 4 h, and, then was no leaching, suggesting a high stability of the fct Pt–Fe/C sample. Itoh et al.83 observed that the phase transitions from disordered fcc to ordered fct phase gives rise to an inhibition of the dissolution of Fe from Pt–Fe nanoparticles. Hoshi et al.84 studied the dissolution of unsupported equimolar Pt–M (M: Cu, Co, Ni, Fe) alloys under conditions of (1) immersion, (2) potentiostatic polarization, and (3) potential cycling in a H2SO4 solution at 25 °C. In 3 h immersion tests, selective dissolution of M atoms occurred immediately, then was quickly suppressed. The rest potential shifted rapidly in the noble direction and approached that for pure Pt. The results indicated that the surfaces of these alloys were covered with a Pt-enriched layer due to preferential dissolution of M atoms. The Pt–Fe alloy showed the largest value, but dissolution was drastically suppressed at the initial stage, as in the other alloys. On the other hand, the amount of Pt ions dissolved from Pt–M alloys after 3 h-immersion test was almost 100 times smaller than the amounts of M ions. Next, the stability of the Pt-enriched layer was examined under potentiostatic conditions at 1.0, 1.2 and 1.4 V vs. SHE for 1 h. When the Pt–M alloys were held at 1.0 and 1.2 V for 1 h, the dissolution rate of both Co and Ni was about 5 ng h−1, which is not higher than that under the steady state in the immersion test. The dissolution rate for Fe was 120 ng h−1, may be higher than that under the steady state in the immersion test, although the dissolution rate could not be accurate. The dissolution of Co, Ni, and Fe at 1.4 V was markedly greater than that at 1.0 and 1.2 V, while Fe dissolution showed little dependence on potential. Finally, potential cycling on the Pt-enriched layer was carried out in a H2SO4 solution at 100 mV s−1 between 0.0 V and 1.4 V. The M dissolution was more significant under potential cycling than by potentiostatic polarization at 1.4 V for 1 h. The amounts of dissolved Co, Ni, and Fe were 17, 29, and 128 ng, respectively, under potentiostatic conditions and 142, 167, and 1440 ng, respectively, under potential cycling (the amount of M loss depends on potential range85). These results indicated that the inhibition due to the Pt-enriched layer did not occur under potential cycling between 0 and 1.4 V. The ECSAs for Pt and the Pt–M alloys were plotted as the ratio to the geometrical surface area (Fig. 7). The ratio was about 3 for all the samples at the initial cycle; it does not change with the number of cycles for the Pt–Cu, Pt–Co, and Pt–Ni alloys, but continues to increase until the 100th cycle for the Pt–Fe alloy.
1), they are not stable under potential cycling.84,85 Firstly, Shukla et al.82 suspended a fct Pt–Fe/C catalyst in a H2SO4 solution at 85 °C for 16 h. Little difference in the XRD patterns of this catalyst before and after test in H2SO4 was observed. They found that only 0.6 ppm of Fe leached from the alloy sample during the first 4 h, and, then was no leaching, suggesting a high stability of the fct Pt–Fe/C sample. Itoh et al.83 observed that the phase transitions from disordered fcc to ordered fct phase gives rise to an inhibition of the dissolution of Fe from Pt–Fe nanoparticles. Hoshi et al.84 studied the dissolution of unsupported equimolar Pt–M (M: Cu, Co, Ni, Fe) alloys under conditions of (1) immersion, (2) potentiostatic polarization, and (3) potential cycling in a H2SO4 solution at 25 °C. In 3 h immersion tests, selective dissolution of M atoms occurred immediately, then was quickly suppressed. The rest potential shifted rapidly in the noble direction and approached that for pure Pt. The results indicated that the surfaces of these alloys were covered with a Pt-enriched layer due to preferential dissolution of M atoms. The Pt–Fe alloy showed the largest value, but dissolution was drastically suppressed at the initial stage, as in the other alloys. On the other hand, the amount of Pt ions dissolved from Pt–M alloys after 3 h-immersion test was almost 100 times smaller than the amounts of M ions. Next, the stability of the Pt-enriched layer was examined under potentiostatic conditions at 1.0, 1.2 and 1.4 V vs. SHE for 1 h. When the Pt–M alloys were held at 1.0 and 1.2 V for 1 h, the dissolution rate of both Co and Ni was about 5 ng h−1, which is not higher than that under the steady state in the immersion test. The dissolution rate for Fe was 120 ng h−1, may be higher than that under the steady state in the immersion test, although the dissolution rate could not be accurate. The dissolution of Co, Ni, and Fe at 1.4 V was markedly greater than that at 1.0 and 1.2 V, while Fe dissolution showed little dependence on potential. Finally, potential cycling on the Pt-enriched layer was carried out in a H2SO4 solution at 100 mV s−1 between 0.0 V and 1.4 V. The M dissolution was more significant under potential cycling than by potentiostatic polarization at 1.4 V for 1 h. The amounts of dissolved Co, Ni, and Fe were 17, 29, and 128 ng, respectively, under potentiostatic conditions and 142, 167, and 1440 ng, respectively, under potential cycling (the amount of M loss depends on potential range85). These results indicated that the inhibition due to the Pt-enriched layer did not occur under potential cycling between 0 and 1.4 V. The ECSAs for Pt and the Pt–M alloys were plotted as the ratio to the geometrical surface area (Fig. 7). The ratio was about 3 for all the samples at the initial cycle; it does not change with the number of cycles for the Pt–Cu, Pt–Co, and Pt–Ni alloys, but continues to increase until the 100th cycle for the Pt–Fe alloy.
 | ||
| Fig. 7 The ECSAs for Pt and fct Pt–M alloys plotted as the ratio to geometrical surface area. Reproduced from ref. 84, copyright 2011, with permission from Elsevier. | ||
The increase of the ORR activity with the degree of ordering of carbon supported Pt–M (M = Fe, Co, Ni, and Cu) catalysts was firstly observed by Xiong and Manthiram.86 Both the Pt–Fe/C and Pt–Co/C catalysts presented an ordered fct structure, while the Pt–Ni/C and Pt–Cu/C catalysts were in a disordered fcc form. The ordered Pt–Fe/C and Pt–Co/C catalysts showed higher ORR activity than the disordered Pt–Ni/C and Pt–Cu/C catalysts. The relationship between the ORR activity and the extent of ordering for the fct Pt–Fe alloys is shown in Fig. 8, the different degree of ordering being obtained by reheating the fct alloy at various temperatures. As can be seen in Fig. 9, the ORR activity increases with increasing the extent of ordering. Ordered fct Pt–Fe catalysts are commonly obtained by thermal treatment of disordered fcc Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalysts. Fct Pt–Fe catalysts presented higher ORR activity and durability than parent fcc Pt–Fe (1
1) catalysts. Fct Pt–Fe catalysts presented higher ORR activity and durability than parent fcc Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalysts.87–90 Considering that an increase of particle size occurs during thermal treatment, the higher specific activity of the fct Pt–Fe than that of fcc Pt–Fe could be ascribed not only to a crystal structure effect but also to a particle size effect.91 Li et al.87 compared the ORR activity of Pt, fcc, partially and fully alloyed fct Pt–Fe nanoparticles. The kinetic current densities were calculated from the ORR polarization curves according to the Koutecky–Levich equation and were used to obtain the specific activity and mass activity (MA, activity massPt−1) (Fig. 9A and B) of the different catalysts. As can be seen in Fig. 9, the specific and mass activities increased in the following order: commercial Pt < fcc Pt–Fe < partially ordered fct Pt–Fe < fully ordered fct Pt–Fe. The H2O2 content in ORR catalyzed by the fully ordered fct Pt–Fe was 1%, attesting the high selectivity of these Pt–Fe NPs on catalyzing ORR via a four-electron process. The fully ordered fct Pt–Fe NPs also showed remarkable durability in the ORR test condition. Moreover, when suspended in a HNO3 solution for 1 h, the fully ordered fct Pt–Fe NPs showed a very small Fe/Pt composition change from 52/48 to 50/50, but the partially ordered fct Pt–Fe NPs had their Fe/Pt composition decreased to 41/59. Thus, the Fe layer sandwiched by two Pt layers in the fct Pt–Fe was greatly stabilized by strong d-orbital interactions between Fe and Pt.
1) catalysts.87–90 Considering that an increase of particle size occurs during thermal treatment, the higher specific activity of the fct Pt–Fe than that of fcc Pt–Fe could be ascribed not only to a crystal structure effect but also to a particle size effect.91 Li et al.87 compared the ORR activity of Pt, fcc, partially and fully alloyed fct Pt–Fe nanoparticles. The kinetic current densities were calculated from the ORR polarization curves according to the Koutecky–Levich equation and were used to obtain the specific activity and mass activity (MA, activity massPt−1) (Fig. 9A and B) of the different catalysts. As can be seen in Fig. 9, the specific and mass activities increased in the following order: commercial Pt < fcc Pt–Fe < partially ordered fct Pt–Fe < fully ordered fct Pt–Fe. The H2O2 content in ORR catalyzed by the fully ordered fct Pt–Fe was 1%, attesting the high selectivity of these Pt–Fe NPs on catalyzing ORR via a four-electron process. The fully ordered fct Pt–Fe NPs also showed remarkable durability in the ORR test condition. Moreover, when suspended in a HNO3 solution for 1 h, the fully ordered fct Pt–Fe NPs showed a very small Fe/Pt composition change from 52/48 to 50/50, but the partially ordered fct Pt–Fe NPs had their Fe/Pt composition decreased to 41/59. Thus, the Fe layer sandwiched by two Pt layers in the fct Pt–Fe was greatly stabilized by strong d-orbital interactions between Fe and Pt.
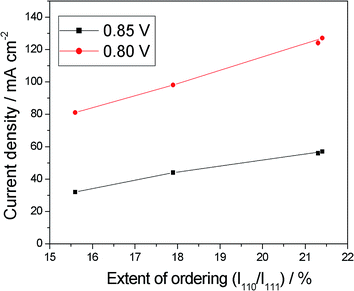 | ||
| Fig. 8 Relationship between the catalytic activity and the extent of ordering, expressed as the 110 to 111 XRD peak intensity ratio (I110/I111) for Pt–Fe/C. Reprinted from ref. 86, copyright 2005, with permission from The Electrochemical Society. | ||
 | ||
| Fig. 9 (A) Specific activities of different catalysts at 0.9 V. (B) Mass activities of different catalysts at 0.9 V. Reproduced from ref. 87, copyright 2015, with permission from ACS. | ||
However, as can be seen in Fig. 4C, among different compositions of fcc Pt–Fe catalysts, the fcc Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is not the best. For the fct Pt–Fe/C catalyst a better comparison is with the ordered fcc Pt–Fe/C (3
1) is not the best. For the fct Pt–Fe/C catalyst a better comparison is with the ordered fcc Pt–Fe/C (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalyst.13,65 The values of the fct Pt–Fe/C to fcc Pt3Fe/C ORR specific activity ratio (SAfct PtFe/SAfcc Pt3Fe) were 1.15 (ref. 13) and 1.11,65 indicating that fct Pt–Fe is slightly more active than the ordered fcc Pt–Fe (3
1) catalyst.13,65 The values of the fct Pt–Fe/C to fcc Pt3Fe/C ORR specific activity ratio (SAfct PtFe/SAfcc Pt3Fe) were 1.15 (ref. 13) and 1.11,65 indicating that fct Pt–Fe is slightly more active than the ordered fcc Pt–Fe (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After potential cycling (5k cycles between 0.6 and 1.2 V vs. RHE), however, the ORR SA of ordered fcc Pt3Fe/C was higher than that of fct Pt–Fe/C.65 The difference in ECSA between the Pt–Fe intermetallic compounds following RPC was due to the instability of Fe in the fct Pt–Fe/C catalyst. The leaching of Fe from fct Pt–Fe/C resulted in an increase in the ECSA. Fcc Pt3Fe/C had a greater tolerance to the leaching of Fe than fct Pt–Fe/C. When the Fe was leached, the ECSA of fct Pt–Fe/C increased, but its fct structure was partially destroyed. After 5000 potential cycles, although fct Pt–Fe/C has a higher mass activity than Pt3Fe/C and a commercial Pt/C, fcc Pt3Fe/C showed superior specific activity than fct Pt–Fe/C, and the SA of fct Pt–Fe/C decreased to a value near to that of the commercial Pt/C. After 5000 potential cycles, XRD results revealed the presence of a mixture of two phases, that is, Pt and fct Pt–Fe, in the fct Pt–Fe/C catalyst, indicating the transformation of part of fct Pt–Fe to Pt.
1). After potential cycling (5k cycles between 0.6 and 1.2 V vs. RHE), however, the ORR SA of ordered fcc Pt3Fe/C was higher than that of fct Pt–Fe/C.65 The difference in ECSA between the Pt–Fe intermetallic compounds following RPC was due to the instability of Fe in the fct Pt–Fe/C catalyst. The leaching of Fe from fct Pt–Fe/C resulted in an increase in the ECSA. Fcc Pt3Fe/C had a greater tolerance to the leaching of Fe than fct Pt–Fe/C. When the Fe was leached, the ECSA of fct Pt–Fe/C increased, but its fct structure was partially destroyed. After 5000 potential cycles, although fct Pt–Fe/C has a higher mass activity than Pt3Fe/C and a commercial Pt/C, fcc Pt3Fe/C showed superior specific activity than fct Pt–Fe/C, and the SA of fct Pt–Fe/C decreased to a value near to that of the commercial Pt/C. After 5000 potential cycles, XRD results revealed the presence of a mixture of two phases, that is, Pt and fct Pt–Fe, in the fct Pt–Fe/C catalyst, indicating the transformation of part of fct Pt–Fe to Pt.
Finally, an interesting nanostructured Pt–Fe catalyst was prepared by Lee et al.92 Support-free intermetallic FePt nanotubes (fct Pt–Fe NTs) were fabricated by an electrospinning process. Fct Pt–Fe NTs showed higher performance than commercial Pt/C; they also showed excellent durability after an accelerated durability test at a potential of 1.4 V, at which carbon corrosion occurs.
The enhanced catalytic activity of ordered fct Pt–Fe catalysts was explained by an optimum number of Pt and Fe nearest neighbors, d-electron density in Pt and Pt–Pt distance. More recently, lattice strain was considered to play an important role in the oxygen reduction catalysis on Pt-based catalysts. However, so far, direct evidence of the lattice strain in the catalyst nanoparticles has not been achieved. By using aberration-corrected high-resolution transmission electron microscopy combined with image simulations, a unique core–shell structure, that is, a percolated lattice-contracted Pt–Fe alloy core and a Pt-rich surface with a gradient compressive strain, was directly demonstrated within individual dealloyed Pt–Fe nanoparticles and thus provides direct evidence for the strain effect on their enhanced oxygen reduction activity.93 Zhang et al.90 showed that surface Pt strain in the core/shell Pt–Fe/Pt NPs with Pt in three atomic layers can be rationally tuned via core structural transition from fcc to fct structure. The high activity observed from the fct Pt–Fe/Pt NPs for the ORR is due to the release of the overcompressed Pt strain by the fct Pt–Fe, as suggested by quantum mechanics–molecular mechanics (QM–MM) simulations.
3.6 Oxygen reduction on ternary Pt–Fe–M catalysts
To further improve the catalytic activity for oxygen reduction of binary Pt-based catalysts, ternary catalysts formed by platinum alloyed with various first row transition metals have been investigated.25 Generally, Pt–Fe–M materials form a ternary alloy. Pt–Fe–M materials with M = Cu,13,90,94–97 Co,96,98–102 Ni96,102–109 and V104,109–113 were the most studied Pt–Fe-based ternary catalysts for the ORR. These alloys were both in a disordered fcc96,98,101 and in ordered fcc94,95,99,103–105 and fct94,95,97,99,100,102 forms. Generally, the ORR activity of Pt–Fe-based ternary catalysts depends on the chemical and structural characteristics of the alloy, such as the type of the third metal,96 the relative amount of alloy components,98 the crystal structure99 and the degree of ordering.94 The dependence of the ORR specific activity of carbon-supported disordered fcc Pt–Fe–M (M = Cr, Mn, Co, Ni and Cu) (Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Fe + M) = 1
(Fe + M) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalysts on the lattice parameter was reported by Shim et al.96 As shown in Fig. 10, the ORR SA/lattice parameter plot presented a volcano curve, similar to the plot of the ORR activity vs. the electronic and geometric parameters reported by Mukerjee et al.41 The Pt–Fe–Co catalyst showed the maximum value of specific activity. The SA based on the oxide reduction area showed the same trend compared to the specific activity by hydrogen desorption. Zhong et al.109 found that the ORR mass activity of fcc Pt–Fe–Ni (31
1) catalysts on the lattice parameter was reported by Shim et al.96 As shown in Fig. 10, the ORR SA/lattice parameter plot presented a volcano curve, similar to the plot of the ORR activity vs. the electronic and geometric parameters reported by Mukerjee et al.41 The Pt–Fe–Co catalyst showed the maximum value of specific activity. The SA based on the oxide reduction area showed the same trend compared to the specific activity by hydrogen desorption. Zhong et al.109 found that the ORR mass activity of fcc Pt–Fe–Ni (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34) was higher than that of fcc Pt–Fe–V (32
34) was higher than that of fcc Pt–Fe–V (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14), but the ORR specific activity was quite similar. The ORR activity of ordered fcc Pt–Fe–M/C (M = Ni and V) catalysts was higher than that of the corresponding binary fcc Pt–Fe/C catalysts.104,109 For disordered fcc Pt–Fe–Co alloys, the ORR mass activity of the catalysts with Pt
14), but the ORR specific activity was quite similar. The ORR activity of ordered fcc Pt–Fe–M/C (M = Ni and V) catalysts was higher than that of the corresponding binary fcc Pt–Fe/C catalysts.104,109 For disordered fcc Pt–Fe–Co alloys, the ORR mass activity of the catalysts with Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co compositions (94
Co compositions (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (85
1) and (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was higher than that of Pt–Fe (97
5) was higher than that of Pt–Fe (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), while for other Pt
3), while for other Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co compositions it was lower than that of Pt–Fe.98
Co compositions it was lower than that of Pt–Fe.98
 | ||
| Fig. 10 Specific activity of the Pt alloy catalysts on the lattice parameter based on the hydrogen desorption area (white symbol) and the oxide reduction area (black symbol) calculated by cyclic voltammogram. (Circle) Pt/C, (square) Pt–Fe–Cr/C, (triangle up) Pt–Fe–Mn/C, (triangle down) Pt–Fe–Co/C, (diamond) Pt–Fe–Ni/C, (hexagon) Pt–Fe–Cu/C. The specific activity normalized with respect to the electrochemically active surface area. Reproduced from ref. 96, copyright 2000, with permission from Elsevier. | ||
Regarding the tetragonal Pt–Fe–M catalysts, two different type of fct Pt–Fe–M structures can be obtained, that is, (1) for Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Fe + M) compositions = 1
(Fe + M) compositions = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Fe atoms are substituted partially by M atoms in lattice planes corresponding to the (1/2, 0, 1/2) and (0, 1/2, 1/2) crystallographic sites of fct Pt–Fe, while only Pt is present in the (0, 0, 0) and (1/2, 1/2, 0) sites, and (2) for Pt
1 Fe atoms are substituted partially by M atoms in lattice planes corresponding to the (1/2, 0, 1/2) and (0, 1/2, 1/2) crystallographic sites of fct Pt–Fe, while only Pt is present in the (0, 0, 0) and (1/2, 1/2, 0) sites, and (2) for Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M compositions = 1 − x
M compositions = 1 − x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 − y
1 − y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x + y M is present in every plane, while the Pt and Fe atoms are present in alternate planes. For example, the crystal structure of stoichiometric tetragonal Pt2FeCu has the (0, 0, 0) and (1/2, 1/2, 0) sites occupied by Pt atoms and the (1/2, 0, 1/2) and (0, 1/2, 1/2) sites occupied by either Cu or Fe atoms in a random manner.114 In the case of fct Pt–Fe–Cu catalysts, the specific activity of both the Pt
x + y M is present in every plane, while the Pt and Fe atoms are present in alternate planes. For example, the crystal structure of stoichiometric tetragonal Pt2FeCu has the (0, 0, 0) and (1/2, 1/2, 0) sites occupied by Pt atoms and the (1/2, 0, 1/2) and (0, 1/2, 1/2) sites occupied by either Cu or Fe atoms in a random manner.114 In the case of fct Pt–Fe–Cu catalysts, the specific activity of both the Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu composition (1
Cu composition (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5)70 and (0.92
0.5)70 and (0.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.92
0.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.16)13 was ca. 25% higher than that of fct Pt–Fe. The SA of ordered fct Pt–Fe–M (2.1
0.16)13 was ca. 25% higher than that of fct Pt–Fe. The SA of ordered fct Pt–Fe–M (2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalysts was 135 (M = Cu),97 133 (M = Co)102 and 137 μA cm−2 (M = Ni),102 that is, almost independent of M. Cho et al.94,95 investigated the ORR activity of carbon-supported Pt–Fe–Cu ternary alloy catalysts in the atomic ratio fcc (6
1) catalysts was 135 (M = Cu),97 133 (M = Co)102 and 137 μA cm−2 (M = Ni),102 that is, almost independent of M. Cho et al.94,95 investigated the ORR activity of carbon-supported Pt–Fe–Cu ternary alloy catalysts in the atomic ratio fcc (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and fct (2
1) and fct (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As observed for fct Pt–Fe,86,87 also the ORR activity of both fcc Pt6–Fe–Cu and fct Pt2–Fe–Cu catalysts increased with increasing the degree of ordering. An increase of the ORR activity with increasing the degree of ordering was also observed by Chen et al.13 for fct Pt–Fe–Cu/C (1
1). As observed for fct Pt–Fe,86,87 also the ORR activity of both fcc Pt6–Fe–Cu and fct Pt2–Fe–Cu catalysts increased with increasing the degree of ordering. An increase of the ORR activity with increasing the degree of ordering was also observed by Chen et al.13 for fct Pt–Fe–Cu/C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17) catalysts. Tamaki et al.99 observed that the mass activity of ordered fct Pt2FeCo/C is about twice as high as that of ordered fcc P6FeCo/C. In addition to a more suitable geometric and electronic arrangements, the improvement in the ORR activity of ternary alloy catalysts than that of Pt–Fe could be ascribed to more favoured release of Pt strain in the fct Pt–Fe–M/Pt than in the fct Pt–Fe/Pt, as calculated by QM–MM simulations.90
0.17) catalysts. Tamaki et al.99 observed that the mass activity of ordered fct Pt2FeCo/C is about twice as high as that of ordered fcc P6FeCo/C. In addition to a more suitable geometric and electronic arrangements, the improvement in the ORR activity of ternary alloy catalysts than that of Pt–Fe could be ascribed to more favoured release of Pt strain in the fct Pt–Fe–M/Pt than in the fct Pt–Fe/Pt, as calculated by QM–MM simulations.90
The electrochemical stability of ternary Pt–Fe–M catalysts was investigated by RPC in acid media.99,100,102 The stability of a fcc Pt–Fe–V/C catalyst was evaluated by comparing its ORR activity before and after 5k cycles between 0.45 and 1.05 V vs. RHE in a HClO4 solution.112 This catalyst after 5000 cycles showed about 20% increase in mass activity, indicating a good electrochemical stability. Tamaki et al.99 compared the ORR stability of ordered fcc Pt6FeCo/C and fct Pt2FeCo/C catalysts by RPC between 0.6 and 1.0 V vs. RHE. Although the decrease of the mass activity following 5k cycles was the same for both catalysts (ca. 25%), the cycling effect on the specific activity of these catalysts, calculated by the MA/ECSA ratio, was different: indeed, after 5k cycles the SA of Pt6FeCo was almost stable (4% decrease), whereas the SA of Pt2FeCo showed a decrease of 37%. The high stability of the Pt6FeCo catalyst was likely due to the formation of a Pt skin, protecting the underlying bulk alloy from corrosion, as observed for the fcc Pt3Fe catalyst. Following 10k cycles, the ratios of Fe/Pt and Co/Pt decreased more for Pt2FeCo (ca. 65% for both metals) than for Pt6FeCo (ca. 32 and 12%, respectively). When Fe was leached, the ECSA of fct Pt2FeCo increased, but its fct structure was partially destroyed. The comparison of XRD patterns of the Pt2FeCo catalyst, initially and after 10k cycles, showed that after the durability test, a new peak appeared next to the (111) reflexion of fct Pt2FeCo, corresponding to the (111) reflexion of pure Pt, thus indicating the transformation of part of ordered Pt2FeCo to Pt. Arumugam et al.100 carried out durability measurements on unsupported ordered fct Pt–Fe–Cu, fct Pt–Fe–Co and fct Pt–Fe catalysts with similar initial mass activity, by RPC between 0.6 and 1.0 V vs. RHE at 60 °C. The durability of mass activities for these catalysts are shown in Fig. 11. The retention in mass activity of fct Pt–Fe–Cu was higher than that of the other two catalysts, fct Pt–Fe and fct Pt–Fe–Co. This higher retention in mass activity of fct Pt–Fe–Cu agree with the higher retention in ECSA of the catalyst. For the ordered fct Pt–Fe–Cu catalyst, the degradation in the half wave potential was only 15 mV over 10k durability cycles with retention of 70% of its initial MA, while for the fct Pt–Fe–Co and fct Pt–Fe catalysts the E1/2 decrease was significantly higher, i.e., 26 and 30 mV, with retention of 60 and 40% of the initial MA, respectively. The synergistic effect of the ordered structure and Cu with higher standard redox potential hindered dissolution of Fe and Cu from the catalyst, helping to retain the ordered structure in the fct Pt–Fe–Cu catalyst, accounting for the enhanced durability in ECSA and mass activity.
 | ||
| Fig. 11 (A) Mass activity and (B) retention in mass activity for catalysts for different number of durability cycles at 60 °C. Reproduced from ref. 97, copyright 2015, with permission from ACS. | ||
4. Methanol oxidation on Pt–Fe and Pt–Fe–M catalysts
4.1 General overview
Methanol oxidation is a slow reaction requiring multiple sites for the adsorption of methanol and sites that can donate OH species for desorption of the adsorbed methanol oxidation intermediates.115–117 The main reaction product is CO2, although significant amounts of formaldehyde, formic acid and methyl formate were also detected. The methanol oxidation reaction can proceed according to multiple mechanisms, however, it is widely accepted that the most significant reactions are the adsorption of methanol and the oxidation of CO, according to this simplified reaction mechanism:| CH3OH → (CH3OH)ads | (6) |
| (CH3OH)ads → (CO)ads + 4H+ + 4e− | (7) |
| (CO)ads + H2O → CO2 + 2H+ + 2e− | (8) |
Platinum is the most active metal for dissociative adsorption of methanol, but at room or moderate temperatures it is readily poisoned by carbon monoxide, a by product of methanol oxidation. To overcome this drawback, many binary and ternary Pt-based electrocatalysts have been investigated, and, among them, binary Pt–Ru and ternary Pt–Ru-based catalysts were the most promising.25,118,119 According to the bifunctional mechanism,120 the CO-poisoned Pt is regenerated via a surface reaction between Pt-adsorbed CO and Ru-adsorbed oxygenated species to yield CO2. According to the ligand model,117,120 instead, the change in Pt electronic properties by the presence of Ru makes Pt atoms more inclined to OH adsorption120 or even to dissociative adsorption of methanol.118 Fe represents a more plentiful, lower cost, and reasonably less toxic alternative as compared with Ru. So, due to the attractiveness of Fe as a viable catalytic component and a potential replacement for Ru, Pt–Fe and Pt–Ru–Fe materials have been investigated as catalysts for the MOR.
4.2 Methanol oxidation on binary Pt–Fe catalysts
One approach for discovering effective electrocatalysts is to rapidly evaluate large libraries of potential candidates. Promising materials identified during this preliminary screening step can then be subjected to more extensive and quantitative testing. Eighteen Pt–M binary (M = Sn, Ta, W, Mo, Ru, Fe, In, Pd, Hf, Zn, Zr, Nb, Sc, Ni, Ti, V, Cr, Rh) thin film composition spreads were deposited at low M concentrations using magnetron sputtering and screened for methanol electrooxidation activity using a fluorescence assay.121 The electrochemical fluorescence assay revealed highest activity in the films with M = Sn, Zn, In, Fe, and Ru. Pt–Fe showed the best activity at 10 at% Fe.Comparison of the MOR activity of large scale Pt–M (M = Fe, Co, Ni) catalysts was carried out.122–124 This comparison resulted in conflicting results for fcc and fct Pt–Fe catalysts. The MOR activity of carbon nanotube supported fcc Pt–M catalysts was in the order Pt–Co > Pt–Ni > Pt–Fe.122,123 Conversely, fct Pt–Fe nanoparticles prepared by a reverse microemulsion method showed enhanced MOR activity compared with Pt–Co and Pt–Ni catalysts prepared by the same method.124
Lee et al.125 investigated the activity for methanol oxidation of binary Pt–M (M = Fe, Ru and Mo) ternary Pt–Ru–Fe and Pt–Ru–Mo catalysts via combinatorial synthesis and high-throughput screening method. For Pt–Fe binary compositions with the Fe atomic fraction in the range 28–97 at% Fe, they observed higher MOR activity than Pt for Fe content in the range 28–60 at%. However, when Fe loading was higher than 60 at%, the MOR activity decreased drastically and was lower than pure Pt.
The MOR activity of large scale Pt–Fe alloys was extensively investigated.122–124,126–141 The dependence of the fcc Pt–Fe to Pt MOR activity ratio (APt–Fe/APt) in acid media on Fe content in the catalyst by different works123,126–131 is shown in Fig. 12. In agreement with the combinatorial synthesis and high-throughput screening method previously reported,104 for iron contents <60 at% Fe, the MOR activity of Pt–Fe was higher than that of Pt. Considering that the MOR activity is generally expressed as the current density per geometric area, the effect of Fe content could be related to both the specific activity and the active area. As can be seen in Fig. 12, the trend is plotted by an order 4 parabolic fit that identifies the peak (APt–Fe/APt) ratio at ca. 17 at% Fe, in acceptable agreement with electrochemical fluorescence assays (the highest activity at 10 at% Fe),121 and in very good agreement with MOR measurements in alkaline media (best activity at 19 at% Fe).132 In all these works, the catalytic activity of Pt–Fe was compared with pure Pt. In few cases the MOR activity of Pt–Fe was compared with state-of-the-art Pt–Ru/C. Scofield et al.133 found that the MOR activity of Pt–Ru (45 at% Ru) was more than 7 times higher than that of Pt–Fe (56 at% Fe). Conversely, the electrochemical activity of Pt–Fe, in the form of graphene supported Pt–Fe nanotubes, was much higher than the commercial Pt–Ru/C catalyst.134 This result, however, could be influenced by the different morphology and the different support of the catalyst. The improvement of the MOR activity of Pt by Fe presence was explained by the bifunctional mechanism and by electronic effects. On the one hand, the addition of Fe promotes the OH species adsorption on the Pt–Fe surface at low potentials, thus, enhancing the MOR activity.127 On the other hand, the strong modification in the electronic structure of the Pt skin by the underlying Fe lowered the Fermi level at the alloy surface. The skin showed less electronic density in the d band. The lowered electron density of the d orbital of Pt weakened the electron back-donation from Pt to CO, leading to a decreased CO poisoning and an improved MOR activity.135,136 Moreover, adding Fe to Pt/C catalyst can improve the dispersion of Pt particles and its electrochemically active surface area, thus enhancing its electrocatalytic activity and stability.137,138 The effect of alloying degree of the fcc structure on the MOR was not evaluated, but the effect of the crystal structure was investigated. Wen et al.126 and Zeng et al.139 compared the MOR activity of fcc and fct Pt–Fe catalysts. Heat treatment transformed the as-synthesized disordered fcc Pt–Fe nanoparticles into chemically ordered fct structure. They observed that annealing treatment reduced the electrochemical activity. The MOR current densities normalized by Pt mass of as-prepared fcc and thermally treated at 700 and 800 °C fct Pt–FeC catalysts are shown in Fig. 13. The as-prepared Pt–Fe/C catalyst was more active than both heat-treated catalysts. Following thermal treatment, the negative effect of the decrease in surface area due to particle size increase prevailed on the positive effect (if any) of the crystal structure transition. Thus, the mass activity of fcc Pt–Fe was higher than that of fct Pt–Fe. The heat-treated catalysts, however, showed higher specific activities compared to the as-prepared one: the higher SA could be ascribed to the crystal structure effect, but could also be due to a particle size effect.91
 | ||
| Fig. 12 Dependence of the fcc Pt–Fe to Pt MOR activity ratio (APt–Fe/APt) in acid media on Fe content in the catalyst by different literature data in ref. 126–131. | ||
 | ||
| Fig. 13 Room temperature cyclic voltammograms of as-prepared fcc and thermally treated at 700 and 800 °C fct Pt–FeC catalysts for methanol electrooxidation in 0.5 M CH3OH + 0.1 M H2SO4 at a scan rate of 20 mV s−1. Reproduced from ref. 139, copyright 2007, with permission from Elsevier. | ||
Finally, Fe2O3(Fe3O4)@Pt core–shell nanostructures were investigated as catalysts for methanol oxidation.142,143 Liu et al.142 synthesized Fe2O3/@Pt core–shell nanoparticles with amorphous Fe2O3 cores. The Fe/Pt atomic ratios used were varied from 1/1 to 4/1. The presence of amorphous iron oxide improved the dispersion of Pt particles, resulting in a higher ECSA than pure Pt. The dependence of the Fe2O3/@Pt to Pt MOR activity ratio (APt–Fe/APt) in acid media on Fe/Pt atomic ratio is shown in Fig. 14. The mass activity of all Fe2O3/@Pt catalysts was considerably higher than that of the Pt/C catalyst. MA of the Fe2O3/Pt nanoparticles went through a maximum at Fe/Pt = 2.4, whereas the ECSA increased with increasing Fe/Pt ratio. This is due to the dependence of the specific activity on Fe/Pt ratio, going through a maximum at Fe/Pt ca. 2. The enhanced SA of the Fe2O3/@Pt catalysts was associated with electronic effects between Fe2O3 and Pt. For Fe/Pt >2, however, SA decreased with increasing Fe content in the catalyst, due to the decrease of Pt atoms per unit area. The forward to reverse scan peak current ratio (jf/jb) of all Fe2O3/@Pt catalysts was also higher than that of the Pt/C catalyst, indicating that Fe2O3/@Pt catalysts have better tolerance to intermediates than the Pt/C catalyst. The electrochemical oxidation of the carbonaceous intermediate can also be promoted by the amorphous Fe2O3 cores. For Fe2O3/@Pt with Fe/Pt 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RPC tests showed a slowly decrease of the ECSA with increasing scan cycles, maintaining values about 86% and 89% of the initial value after 6000 potential cycles. Conversely, the ECSA of Fe2O3/Pt (4
1, RPC tests showed a slowly decrease of the ECSA with increasing scan cycles, maintaining values about 86% and 89% of the initial value after 6000 potential cycles. Conversely, the ECSA of Fe2O3/Pt (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Pt/C decreases to about 66% and 59% of the initial value. This result indicates that the structural stability of the prepared core–shell nanoparticles is much higher than that of the Pt/C catalyst. The core–shell architecture with an amorphous Fe2O3 core prevents Pt dissolution, nanoparticle aggregation, and loss of contact to the carbon black support compared with the Pt/C catalyst. Conversely, the significant decrease in ECSA in the Fe2O3/Pt (4
1) and Pt/C decreases to about 66% and 59% of the initial value. This result indicates that the structural stability of the prepared core–shell nanoparticles is much higher than that of the Pt/C catalyst. The core–shell architecture with an amorphous Fe2O3 core prevents Pt dissolution, nanoparticle aggregation, and loss of contact to the carbon black support compared with the Pt/C catalyst. Conversely, the significant decrease in ECSA in the Fe2O3/Pt (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalyst has to be ascribed to the dissolution of amorphous Fe2O3 because the Pt shell is too thin to completely cover the amorphous Fe2O3 core. Sánchez-Padilla et al.143 synthesized M@Pt core–shell nanostructures (where M = Ru, Fe3O4, Pd) with a nominal M
1) catalyst has to be ascribed to the dissolution of amorphous Fe2O3 because the Pt shell is too thin to completely cover the amorphous Fe2O3 core. Sánchez-Padilla et al.143 synthesized M@Pt core–shell nanostructures (where M = Ru, Fe3O4, Pd) with a nominal M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt weight ratio of 1
Pt weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fe/Pt atomic ratio 3.5
1 (Fe/Pt atomic ratio 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). By XRD analysis, a shift of Pt reflexions at higher angles with respect to pure Pt was observed for the core–shell materials, particularly for Fe3O4@Pt, indicating a larger contraction of the Pt lattice when interacting with the iron atoms. The Ru@Pt and the Pt catalysts showed a higher catalytic activity than Fe3O4@Pt. On the basis of these results, it seems that for methanol oxidation amorphous Fe2O3 is a more effective core for Pt shell than polycrystalline Fe3O4.
1). By XRD analysis, a shift of Pt reflexions at higher angles with respect to pure Pt was observed for the core–shell materials, particularly for Fe3O4@Pt, indicating a larger contraction of the Pt lattice when interacting with the iron atoms. The Ru@Pt and the Pt catalysts showed a higher catalytic activity than Fe3O4@Pt. On the basis of these results, it seems that for methanol oxidation amorphous Fe2O3 is a more effective core for Pt shell than polycrystalline Fe3O4.
 | ||
| Fig. 14 Dependence of the Fe2O3/@Pt to Pt MOR activity ratio (APt–Fe/APt) in acid media on Fe/Pt atomic ratio from data in ref. 142. | ||
4.3 Methanol oxidation on ternary Pt–Fe–M catalysts
Comparison of large scale ternary Pt–Ru–M (M = Fe, Co, Ni and Mo) with binary Pt–Ru indicated that the addition of M to Pt–Ru enhances the electrocatalytic activity for methanol oxidation.125,145,146 Among these ternary catalysts, Pt–Ru–Fe showed the highest mass activity, but the lowest specific activity.125,145
Various papers reported the synthesis and MOR activity of ternary Pt–Ru–Fe catalysts.125,133,145–151 Generally, Pt–Ru–Fe catalysts were synthesized by reduction of a mixture of metal precursors with NaBH4.125,145–148 An interesting alternative method to prepare Pt–Ru–Fe catalysts using a chemical replacement reaction in a solution phase was proposed by Wang et al.149,150 Pt–Fe nanocrystals and Ru3+ metal ions were selected to produce PtFe1−xRux nanocrystals. Iron atoms of Pt–Fe lattices were oxidized to Fe2+ and Fe3+ ions and were replaced by Ru atoms by the reduction of Ru3+ ions in solution to form PtFe1−xRux. Most of the Pt atoms were preferentially located in the core region and the Ru atoms were located in the shell region with a intermediate iron layer between the Pt and Ru atoms. The MOR activity of Pt–Ru–Fe catalysts depends on the catalyst composition.125,133 To evaluated the effect of Fe/(Fe + Ru) ratio on the MOR activity, at fixed Pt content in the catalyst, we have plotted the Pt–Ru–Fe to Pt–Ru MOR specific activity ratio (SAPt–Ru–Fe/SAPt–Ru) in acid media against Fe/(Fe + Ru) weight ratio in the catalyst at a fixed Pt/(Fe + Ru) weight ratio of 2.33, using data from ref. 133 The parabolic curve interpolating the experimental points presented a maximum at a value of Fe/(Fe + Ru) of ca. 0.17, as can be observed in Fig. 15A. In the Fe/(Fe + Ru) range 0–0.51, the catalytic activity of Pt–Ru–Fe was higher than that of Pt–Ru. As the Ru content is not constant, the improved catalytic activity could the due to either Fe content increase or Ru content decrease. Indeed, the MOR activity of both Pt–Ru and Pt–Fe catalysts goes through a maximum, at ca. 0.5Ru152 and 0.17Fe (see Fig. 12) atomic fraction, respectively. In these data, in the absence of Fe the Ru atomic fraction in Pt–Ru was 0.45, close to the optimum value. On this basis, if the Ru effect is prevalent, a decrease of the SAPt–Ru–Fe/SAPt–Ru with increasing Fe content (decreasing Ru content) should be observed. Conversely, if the effect of Fe prevails, a maximum should be observed. The maximum in Fig. 15A corresponds to Fe/(Pt + Fe) atomic fraction of ca. 0.17, in excellent agreement with the maximum observed in Fig. 12. Thus, the increase MOR activity in Pt–Ru–Fe has to be fully ascribed to Fe presence. The comparison of the dependence of the Pt–Ru–Fe to Pt MOR activity ratio APt–Ru–Fe/APt and Pt–Fe to Pt MOR activity ratio APt–Fe/APt (curve from Fig. 13) on Fe/Pt weight ratio (Fig. 15B) confirms the effect of Fe. The dependence of both the ratios on Fe/Pt is similar: the maximum activity occurs at close values of Fe/Pt weight ratio (0.06/0.07). The higher values of the APt–Ru–Fe/APt ratio than those of APt–Fe/APt are due to the presence of Ru. The enhanced MOR activity of the Pt–Ru–Fe ternary catalysts was ascribed to the reaction between the weakly adsorbed methanol oxidation intermediate species on the Pt site electronically modified by alloying with Fe and adsorbed water species on the Ru site. The combination of these features resulted in an enhancement in the electro-oxidation of methanol at lower potential.148,150
 | ||
| Fig. 15 (A) Dependence of Pt–Ru–Fe to Pt–Ru MOR specific activity ratio (SAPt–Ru–Fe/SAPt–Ru) in acid media on Fe/(Fe + Ru) weight ratio in the catalyst at a fixed Pt/(Fe + Ru) weight ratio of 2.33, using data from ref. 133. (B) Dependence of the Pt–Ru–Fe to Pt MOR activity ratio APt–Ru–Fe/APt and Pt–Fe to Pt MOR activity ratio APt–Fe/APt (curve from Fig. 13) on Fe/Pt weight ratio. | ||
 | ||
| Fig. 16 CVs of the EOR at Fe3O4@Pt, Pd@Pt, Ru@Pt and Pt-alone in N2-saturated 0.5 M H2SO4 + 0.5 M C2H5OH solution. Scan rate: 20 mV s−1. Reproduced from ref. 143, copyright 2013, with permission from Elsevier. | ||
5. Fe in binary, ternary and quaternary Pt-based catalysts for ethanol oxidation
5.1 General overview
The oxidation mechanism of ethanol in acid solution may be summarized by the following parallel reactions:| CH3CH2OH → [CH3CH2OH]ad → C1ads, C2ads → CO2 (total oxidation) | (9) |
| CH3CH2OH → [CH3CH2OH]ad → CH3CHO → CH3COOH (partial oxidation) | (10) |
The formation of CO2 goes through two adsorbed intermediates C1ads and C2ads, representing fragments with one and two carbon atoms, respectively. C–C bond cleavage to obtain CO2 is a major problem in ethanol electrocatalysis. Platinum surface is quickly poisoned by strongly adsorbed ethanol oxidation intermediate species.9 Efforts to reduce Pt poisoning have been addressed to the addition of co-catalysts, particularly ruthenium and tin, to platinum. Conversely to the methanol oxidation, the best binary catalyst for ethanol oxidation in acid environment is not Pt–Ru but Pt–Sn.9,156 The addition of tin to platinum not only increases the activity of the catalyst towards the oxidation of ethanol and therefore the electrical performance of the DEFC, but also changes the product distribution. As in the case of the methanol oxidation, the enhanced EOR activity of these binary catalysts than that of bare Pt was attributed to the bifunctional effect and to the electronic interaction between Pt and alloyed metals. C–C bond cleavage on Pt, however, is inhibited by Sn presence in both alloyed and non-alloyed catalysts.156 To overcome this drawback, binary and ternary Pt-based electrocatalysts have been investigated.9 In the following part of Section 5 we report the ethanol oxidation reaction on Pt–Fe and, in particular, on multimetal Pt–Fe containing catalysts.
5.2 Ethanol oxidation on binary Pt–Fe catalysts
Few works have been addressed to the ethanol oxidation on Pt–Fe catalysts.157–159 Wang et al.157 deposited iron nanoparticles on a glass carbon electrode by cyclic voltammetry, then Fe core/Pt shell nanoparticles were obtained through galvanic replacement. The Fe@Pt catalyst showed higher electrocatalytical activity than Pt. Dong et al.158 observed that, in comparison to single wall carbon nanotube (SWCNT) supported Pt, Pt–Fe/SWCNT has similar ethanol oxidation onset potential, lower ethanol oxidation forward peak current density, and a comparable ratio of the forward to the reverse peak current density, indicating the catalyst tolerance to CO and other carbonaceous species. The EOR activity of Pt and Pt–M (M = Ag, Ca, Cd, Cs, Cu, Fe, Ir, Mg, Pd, Sn, Zr) bimetallic nanocatalysts, prepared by the polyol method, was investigated by Kivrak et al.159 The electrochemical activity for ethanol oxidation was in the order of Pt–Sn > Pt–Cs > Pt–Cu > Pt–Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Pt–Ca
Pt–Ca![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Pt–Pd > Pt > Pt–Ir > Pt–Mg > Pt–Ag > Pt–Zr.
Pt–Pd > Pt > Pt–Ir > Pt–Mg > Pt–Ag > Pt–Zr.
Two platinum/iron oxide (magnetite) composites were investigated for the ethanol oxidation in a H2SO4 solution.143,160 The effect of Fe3O4 on the EOR activity and stability of carbon nanotube supported Pt was evaluated [160]. Pt–Fe3O4/MWCNT showed a higher EOR activity than that of Pt/MWCNT, with almost 46% increase in current density. Moreover, the magnetite-containing catalysts presented high electrochemical stability, with negligible surface area losses (less than 7%) following 500 cycles between 0.6 and 1.2 V vs. SHE. As previously reported, Sánchez-Padilla et al.143 synthesized M@Pt core–shell nanostructures (where M = Ru, Fe3O4, Pd) with a nominal M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt weight ratio of 1
Pt weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fe/Pt atomic ratio 3.5
1 (Fe/Pt atomic ratio 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Unlike methanol oxidation, the EOR activity of Fe3O4@Pt was higher than that of Pt. As can be seen in Fig. 16, the catalytic activity for the EOR was in the order Ru@Pt ≥ Fe3O4@Pt > Pt ≫ Pd@Pt.
1). Unlike methanol oxidation, the EOR activity of Fe3O4@Pt was higher than that of Pt. As can be seen in Fig. 16, the catalytic activity for the EOR was in the order Ru@Pt ≥ Fe3O4@Pt > Pt ≫ Pd@Pt.
5.3 Ethanol oxidation on ternary and quaternary Fe-containing Pt-based catalysts
As reported in Section 5.1, Pt–Sn is the most effective for the electro-oxidation of ethanol.9,156 To further improve its EOR activity and stability and to promote C–C bond cleavage, the effect of the addition of a third metal to Pt–Sn, has been studied by combinatorial libraries of ternary Pt–Sn–M materials.161 Electrode arrays containing 91 combinations of Pt–Sn–M (M = Fe, Ni, Pd, and Ru) were prepared by borohydride reduction of metal precursors on carbon paper, and screened by fluorescence assay for ethanol electrooxidation. Catalysts that showed the highest EOR activity were Pt–Sn–Fe (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Pt–Sn–Ni (8
1), Pt–Sn–Ni (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Pt–Sn–Pd (7
1), Pt–Sn–Pd (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1) and Pt–Sn–Ru (7
2.1) and Pt–Sn–Ru (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2). These materials were considerably more active than Pt or Pt–Sn catalysts, also present in the electrode arrays. These 4 compositions were synthesized as nanoparticles, showing a Pt fcc structure with an average crystallite size of about 2.0 nm for all catalysts. Chronoamperometry measurements were carried out on these catalysts: as can be seen in Fig. 17, Pt–Sn–Fe presented the highest mass and specific EOR activities, followed by Pt–Sn–Ni, Pt–Sn–Pd and Pt–Sn–Ru. Pt–Sn–Fe also showed the highest PEMFC performance. The presence of a third metal on the Pt–Sn-based compositions can affect the electrocatalytic activity. Fe and Ni can exert an electronic effect on Pt and Sn atoms caused by their low electronegativity, boosting the oxophilic character of the surface and modifying the –OH adsorption energy and the concentration of this species on the catalyst surface, thus improving the water activation on Pt and Sn, resulting in ethanol oxidation at lower potentials.
1.2). These materials were considerably more active than Pt or Pt–Sn catalysts, also present in the electrode arrays. These 4 compositions were synthesized as nanoparticles, showing a Pt fcc structure with an average crystallite size of about 2.0 nm for all catalysts. Chronoamperometry measurements were carried out on these catalysts: as can be seen in Fig. 17, Pt–Sn–Fe presented the highest mass and specific EOR activities, followed by Pt–Sn–Ni, Pt–Sn–Pd and Pt–Sn–Ru. Pt–Sn–Fe also showed the highest PEMFC performance. The presence of a third metal on the Pt–Sn-based compositions can affect the electrocatalytic activity. Fe and Ni can exert an electronic effect on Pt and Sn atoms caused by their low electronegativity, boosting the oxophilic character of the surface and modifying the –OH adsorption energy and the concentration of this species on the catalyst surface, thus improving the water activation on Pt and Sn, resulting in ethanol oxidation at lower potentials.
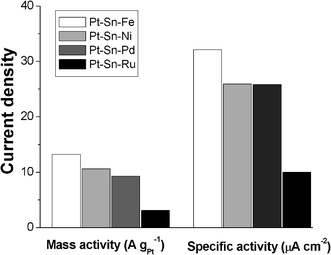 | ||
| Fig. 17 Histogram of the mass activity and specific activity for ethanol oxidation of various Pt–Sn–M catalysts from data in ref. 161. | ||
Ammam et al.162 reported Pt–Mn/C binary alloy catalysts with high catalytic activities toward ethanol oxidation. On this basis, Ammam and Easton163 synthesized ternary Pt–Mn–M (M = Fe, Co, Ni, Cu, Mo, and Sn) catalysts with the same Pt: transition metal ratio. The EOR activity of Pt–Mn–M/C (M = Fe, Co, Ni, Cu and Mo) catalysts was higher than that of binary Pt–Mn/C catalysts, and the activity was in the order Pt–Mn–Cu/C > Pt–Mn–Fe/C > Pt–Mn–Mo/C > Pt–Mn–Ni/C > Pt–Mn–Co/C > Pt–Mn/C > Pt–Mn–Sn/C. Then, the same authors investigated the EOR activity of two series of quaternary Pt–Mn–Cu–M/C (M = Fe, Co, Ni, and Sn) and Pt–Mn–Mo–M/C (M = Fe, Co, Ni, Cu and Sn) catalysts.164 All quaternary showed superior electrocatalytic activity towards ethanol oxidation than that of ternary Pt–Mn–Cu/C and Pt–Mn–Mo/C catalysts.
6. Conclusions
The effect of iron addition as co-catalyst in binary/ternary Pt-based catalysts for oxygen reduction and methanol/ethanol oxidation was evaluated from the analysis of a large number of literature data. The characteristics of Pt–Fe and Pt–Fe–M catalysts for low-temperature fuel cells and the comparison of their catalytic activities with that of the respective benchmark catalyst are reported in Table 1. A positive effect of Fe alloying on the ORR activity of Pt was observed. The specific activity for the ORR of Pt–Fe depends on the amount of Fe alloyed and goes through a maximum at ca. 14 at% Fe, corresponding to the optimum geometric and electronic effects. Considering that only part of iron present in the catalyst is alloyed with Pt, the amount of Fe in the catalyst at the maximum ORR activity is higher than the amount of Fe alloyed. The ORR activity increases going from disordered fcc Pt1–xFex to ordered fcc Pt3Fe to ordered fct Pt–Fe. Generally, the ORR activity of ternary Pt–Fe–M catalysts was higher than that of the corresponding binary Pt–Fe catalysts. The ORR activity of ternary catalysts increases with increasing the degree of ordering and going from the fcc to the fct structure. As in the case of binary Pt-based alloy catalysts, the improvement in the ORR activity on ternary alloy catalysts than on Pt was ascribed to Pt electronic state and nearest neighbor Pt–Pt distance changes by alloy formation, and to more favoured release of Pt strain in the fct Pt–Fe–M/Pt than in the fct Pt–Fe/Pt. Finally, Pt–Fe catalysts showed higher methanol tolerance during the ORR than Pt.| Reaction/benchmark | Catalyst | Activity/stability | Characteristics | Ref. |
|---|---|---|---|---|
| ORR/Pt | Disordered fcc Pt–Fe | Activity Pt–Fe > Pt stability Pt1–xFex > Pt for x < 0.5 | The alloying of Pt with Fe causes shrinkage of the metal lattice and decrease of the Pt–Pt nearest neighbour bond, thus inducing low energy Pt surface sites and enhanced adsorption of oxygen. Electronic effects by formation of a Pt skin | 3 and 41–65 |
| Ordered fcc Pt3Fe | Activity ord fcc Pt3Fe > dis fcc Pt–Fe stability fcc Pt3Fe > Pt | High methanol-tolerance of Pt–Fe than Pt by ensemble effect | 13,56 and 65 | |
| Fct Pt–Fe | Activity fct Pt–Fe > ord fcc Pt3Fe stability ord fcc Pt3Fe > fct Pt–Fe | The enhanced catalytic activity of ordered fct Pt–Fe catalysts was explained by an optimum number of Pt and Fe nearest neighbors, d-electron density in Pt and Pt–Pt distance | 13,65,82–90,92 and 93 | |
| Pt–Fe–M (M = Cu, Co, Ni, V) | Activity Pt–Fe–M > Pt–Fe, fct Pt–Fe–M > fcc Pt–Fe–M stability fct Pt–Fe–M > fct Pt–Fe | Generally, Pt–Fe–M materials form a ternary alloy. The improvement in the ORR activity on ternary alloy catalysts than on Pt was ascribed to Pt electronic state and nearest neighbor Pt–Pt distance changes by alloy formation | 13,90 and 94–113 | |
| MOR/PtRu | Fcc Pt–Fe | Activity Pt–Fe > Pt | Enhanced activity of Pt–Fe catalysts than Pt alone explained by the bifunctional effect of the electrocatalyst towards the dissociative adsorption of methanol on platinum, mainly towards CO adsorbed species, which can be further oxidized into carbon dioxide due to the presence of Fe, allowing a sufficient coverage by OH species at lower potentials than Pt | 122–141 |
| Fct Pt–Fe | MA fcc Pt–Fe > fct Pt–Fe SA fct Pt–Fe > fcc Pt–Fe | 126 and 139 | ||
| Fe2O3(Fe3O4)@Pt | MA Fe2O3/@Pt > Pt MA Fe2O3/@Pt > Pt activity Pt > Fe3O4@Pt | 142 and 143 | ||
| Pt–Fe–Ru | SA Pt–Fe–Ru > Pt–Ru (range Fe/(Fe + Ru) = 0–0.51 wt ratio for Pt–Ru (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) 1)) |
Enhanced MOR activity of the Pt–Ru–Fe ternary catalysts was ascribed to the reaction between the weakly adsorbed methanol oxidation intermediate species on the Pt site electronically modified by alloying with Fe and adsorbed water species on the Ru site | 125,133 and 145–151 | |
| Pt–Fe–M (M = Pd, Co, Cu) | Activity Pt–Fe–M > Pt–Fe | 153–155 | ||
| EOR/PtSn | Pt–Fe | Activity Pt–Sn > Pt–Fe > Pt | Higher activity of ternary electrocatalysts explained by the beneficial synergistic effect between platinum and iron. Fe exerts an electronic effect on Pt and Sn atoms due to its low electronegativity, boosting the oxophilic character of the surface and modifying the –OH adsorption energy and the concentration of this species on the catalyst surface, thus improving the water activation on Pt and Sn, resulting in ethanol oxidation at lower potentials | 157–159 |
| Pt/Fe3O4 | Activity Pt/Fe3O4 > Pt | 142 and 160 | ||
| Pt–Sn–Fe | Activity Pt–Sn–Fe > Pt–Sn | 161 |
The addition of Fe enhances the activity for methanol and ethanol oxidation of Pt, but the Pt–Fe catalysts showed lower MOR and EOR activity than that of the benchmarks Pt–Ru and Pt–Sn, respectively. Regarding methanol and ethanol oxidation, more than the addition of Fe to Pt in binary Pt–Fe catalysts, the most promising use of iron is its addition to the benchmark Pt–Ru (for the MOR) and Pt–Sn (for the EOR). The MOR activity of Pt–Ru–Fe catalysts depends on the catalyst composition: at a fixed Pt/(Ru + Sn) weight ratio of 2.33, for the Fe/(Fe + Ru) weight ratio in the range 0 < Fe/(Fe + Ru) < 0.5 the MOR activity of Pt–Fe–Ru was higher than that of Pt–Ru, with a maximum at a value of Fe/(Fe + Ru) weight ratio of ca. 0.17. The enhanced MOR activity of the Pt–Ru–Fe ternary catalysts was ascribed to the reaction between the weakly adsorbed methanol oxidation intermediate species on the Pt site electronically modified by alloying with Fe and adsorbed water species on the Ru site. On the other hand, among Pt–Sn and various ternary Pt–Sn–M catalysts, Pt–Fe–Sn (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed the highest EOR activity.
1) showed the highest EOR activity.
One of the major problems hindering the large-scale implementation of PEMFC technology is the catalyst performance loss during long time operation. Different mechanisms contribute to catalyst degradation such as metal particle sintering, catalyst dissolution and catalyst support corrosion. In the case of Pt–Fe catalysts the main drawback is Fe loss. The stability of fcc Pt–Fe catalysts was evaluated by potential cycling tests. For the disordered fcc Pt1–xFex catalysts with x < 0.5 and the ordered fcc Pt3Fe catalyst, the ECSA of Pt–Fe/C catalysts decreased more slowly than pure Pt upon cycling, reflecting the formation of a Pt skin layer, which protects the underlying bulk alloy from corrosion. On the other hand, the Pt–Fe/C catalyst with iron content ≥50 at% Fe showed a similar or higher decrease of the ECSA than Pt/C, indicating no formation of a stable Pt skin. Generally, fcc Pt–Fe catalysts showed a higher stability of the ORR activity than Pt, indicating that, in addition to a higher ECSA stability, Fe loss do not appreciably affect the specific activity of these catalysts. As in the case of fcc Pt–Fe (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalysts, ordered fct Pt–Fe catalysts are not stable under potential cycling. When Fe was leached, the ECSA of fct Pt–Fe/C increased, and its fct structure was partially destroyed. After 5000 potential cycles, the specific activity of fct Pt–Fe/C decreased to a value near to that of commercial Pt/C, and the presence of a mixture of two phases, that is, Pt and fct Pt–Fe was revealed, indicating the transformation of part of ordered Pt–Fe to Pt. The stability of fct Pt–Fe can be improved by the addition of a third metal. The retention in mass activity of fct Pt–Fe–M (M = Cu, Co) was higher than that of the fct Pt–Fe catalyst. This higher retention in mass activity of fct Pt–Fe–Cu was in agreement with the higher retention in its ECSA. The synergistic effect of the ordered structure and Cu with higher standard redox potential hindered dissolution of Fe and Cu from the catalyst, helping to retain the ordered structure in the fct Pt–Fe–Cu catalyst, accounting for the enhanced durability in ECSA and mass activity.
1) catalysts, ordered fct Pt–Fe catalysts are not stable under potential cycling. When Fe was leached, the ECSA of fct Pt–Fe/C increased, and its fct structure was partially destroyed. After 5000 potential cycles, the specific activity of fct Pt–Fe/C decreased to a value near to that of commercial Pt/C, and the presence of a mixture of two phases, that is, Pt and fct Pt–Fe was revealed, indicating the transformation of part of ordered Pt–Fe to Pt. The stability of fct Pt–Fe can be improved by the addition of a third metal. The retention in mass activity of fct Pt–Fe–M (M = Cu, Co) was higher than that of the fct Pt–Fe catalyst. This higher retention in mass activity of fct Pt–Fe–Cu was in agreement with the higher retention in its ECSA. The synergistic effect of the ordered structure and Cu with higher standard redox potential hindered dissolution of Fe and Cu from the catalyst, helping to retain the ordered structure in the fct Pt–Fe–Cu catalyst, accounting for the enhanced durability in ECSA and mass activity.
The state of the art fuel cell anode/cathode catalysts are Pt–Ru//Pt (for DMFCs) and Pt–Sn//Pt (for DEFCs). Considering that ternary Pt–Ru–Fe and Pt–Sn–Fe anode catalysts possess higher activity for methanol and ethanol oxidation than benchmarks Pt–Ru and Pt–Sn, respectively, and that the ternary fct Pt–Cu–Fe cathode catalyst has higher activity and stability than Pt, high performance DMFCs and DEFCs having also high stability can be assembled using Pt-based Fe-containing anode/cathode Pt–Ru–Fe/Pt–Fe–Cu (for DMFCs) and Pt–Sn–Fe/Pt–Fe–Cu (for DEFCs) catalysts.
References
- C. Wang, S. Wang, J. Zhang, J. Li, M. Ouyang and J. Wang, Prog. Chem., 2015, 27, 310 Search PubMed
.
- S. K. Kamarudin, F. Achmad and W. R. W. Daud, Int. J. Hydrogen Energy, 2009, 34, 6902 CrossRef CAS
.
- S. P. S. Badwal, S. Giddey, A. Kulkarni, J. Goel and S. Basu, Appl. Energy, 2015, 145, 80 CrossRef CAS
.
- Y.-J. Wang, N. Zhao, B. Fang, H. Li, X. T. Bi and H. Wang, Chem. Rev., 2015, 115, 3433 CrossRef CAS PubMed
.
- R. N. Singh, R. Awasthi and C. S. Sharma, Int. J. Electrochem. Sci., 2014, 9, 5607 Search PubMed
.
- Z. Chen, D. Higgins, A. Yu, L. Zhang and J. Zhang, Energy Environ. Sci., 2011, 4, 3167 CAS
.
- R. Othman, A. L. Dicks and Z. Zhu, Int. J. Hydrogen Energy, 2012, 37, 357 CrossRef CAS
.
- Z. Wang, G. Shi, J. Xia, F. Zhang, Y. Xia, Y. Li and L. Xia, Acta Chim. Sin., 2013, 71, 1225 CAS
.
- E. Antolini, J. Power Sources, 2007, 170, 1 CrossRef CAS
.
- A. Serov and C. Kwak, Appl. Catal., B, 2009, 90, 313 CrossRef CAS
.
- X. Zhao, M. Yin, L. Ma, L. Liang, C. Liu, J. Liao, T. Lu and W. Xing, Energy Environ. Sci., 2011, 4, 2736 CAS
.
- X. Li, L. An, X. Chen, N. Zhang, D. Xia, W. Huang, W. Chu and Z. Wu, Sci. Rep., 2013, 3, 3234 Search PubMed
.
- L. Chen, C. Bock, P. H. J. Mercier and B. R. MacDougall, Electrochim. Acta, 2012, 77, 212 CrossRef CAS
.
- S. Sun, Adv. Mater., 2006, 18, 393 CrossRef CAS
.
- J. Lyubina, O. Gutfleisch, R. Skomski, K.-H. Müller and L. Schultz, Scr. Mater., 2005, 53, 469 CrossRef CAS
.
- L. Peng, E. Ringe, R. P. van Duyne and L. D. Marks, Phys. Chem. Chem. Phys., 2015, 17, 27940 RSC
.
- J. J. Burton and R. S. Polizzotti, Surf. Sci., 1977, 66, 1 CrossRef CAS
.
- M. S. Spencer, Surf. Sci., 1984, 145, 153 CrossRef CAS
.
- R. V. Chepulskii, W. H. Butler, A. van de Walle and S. Curtarolo, Scr. Mater., 2010, 62, 179 CrossRef CAS
.
- S. Yamakawa, R. Asahi and T. Koyama, Surf. Sci., 2014, 622, 65 CrossRef CAS
.
- Z. Shi, J. Zhang, Z. S. Liu, H. Wang and D. P. Wilkinson, Electrochim. Acta, 2006, 51, 1905 CrossRef CAS
.
- A. Damjanovic and V. Brusic, Electrochim. Acta, 1967, 12, 615 CrossRef CAS
.
- E. Antolini, Mater. Chem. Phys., 2003, 78, 563 CrossRef CAS
.
- H. A. Gasteiger, S. S. Kocha, B. Sompalli and F. T. Wagner, Appl. Catal., B, 2005, 56, 9 CrossRef CAS
.
- E. Antolini, Appl. Catal., B, 2007, 74, 337 CrossRef CAS
.
- E. Antolini, ACS Catal., 2014, 4, 1426 CrossRef CAS
.
- A. A. Gewirth and M. S. Thorun, Inorg. Chem., 2010, 49, 3557 CrossRef CAS PubMed
.
- E. Antolini, Energy Environ. Sci., 2009, 2, 915 CAS
.
- A. Morozan, B. Jousselme and S. Palacin, Energy Environ. Sci., 2011, 4, 1238 CAS
.
- B. Wang, J. Power Sources, 2005, 152, 1 CrossRef CAS
.
- Y. Feng and N. Alonso-Vante, Phys. Status Solidi B, 2006, 245, 1792 CrossRef
.
- Z. Chen, D. Higgins, A. Yu, L. Zhang and J. Zhang, Energy Environ. Sci., 2011, 4, 3167 CAS
.
- R. Othman, A. L. Dicks and Z. Zhu, Int. J. Hydrogen Energy, 2012, 37, 357 CrossRef CAS
.
- Y. Zheng, Y. Jiao, M. Jaronec, Y. Jin and S. Z. Qiao, Small, 2012, 8, 3550 CrossRef CAS PubMed
.
- J. K. Norskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jonsson, J. Phys. Chem. B, 2004, 108, 17886 CrossRef CAS
.
- V. Jalan and E. J. Taylor, J. Electrochem. Soc., 1983, 130, 2299 CrossRef CAS
.
- M. T. Paffett, G. J. Berry and S. Gottesfeld, J. Electrochem. Soc., 1988, 135, 1431 CrossRef CAS
.
- B. C. Beard and P. N. Ross, J. Electrochem. Soc., 1990, 137, 3368 CrossRef CAS
.
- T. Toda, H. Igarashi, H. Uchida and M. Watanabe, J. Electrochem. Soc., 1999, 146, 3750 CrossRef CAS
.
- V. Stamenkovic, T. J. Schmidt, P. N. Ross and N. M. Markovic, J. Electroanal. Chem., 2003, 554–555, 191 CrossRef CAS
.
- S. Mukerjee, S. Srinivasan, M. P. Soriaga and J. McBreen, J. Electrochem. Soc., 1995, 142, 1409 CrossRef CAS
.
- Q. Jia, C. U. Segre, D. Ramaker, K. Caldwell, M. Trahan and S. Mukerjee, Electrochim. Acta, 2013, 88, 604 CrossRef CAS
.
- T. Toda, H. Igarashi and M. Watanabe, J. Electroanal. Chem., 1999, 460, 258 CrossRef CAS
.
- W. Li, W. Zhou, H. Li, Z. Zhou, B. Zhou, G. Sun and Q. Xin, Electrochim. Acta, 2004, 49, 1045 CrossRef CAS
.
- W. Chen, J. Kim, S. Sun and S. Chen, J. Phys. Chem. C, 2008, 112, 3891 CAS
.
- W. Li, Q. Xin and Y. Yan, Int. J. Hydrogen Energy, 2010, 35, 2530 CrossRef CAS
.
- H. Duan, Q. Hao and C. Xu, J. Power Sources, 2014, 269, 589 CrossRef CAS
.
- Z. Yan, M. Wang, J. Liu, R. Liu and J. Zhao, Electrochim. Acta, 2014, 141, 331 CrossRef CAS
.
- F. J. Lai, H. L. Chou, L. S. Sarma, D. Y. Wang, Y. C. Lin, J. F. Lee, B. J. Hwang and C. C. Chen, Nanoscale, 2010, 2, 573 RSC
.
- Z. Zhang, M. Li, Z. Wu and W. Li, Nanotechnology, 2011, 22, 015602 CrossRef PubMed
.
- D. Chen, X. Zhao, S. Chen, H. Li, X. Fu, Q. Wu, S. Li, Y. Li, B. L. Su and R. S. Ruoff, Carbon, 2014, 68, 755 CrossRef CAS
.
- D. Y. Wang, H. L. Chou, C. C. Cheng, Y. H. Wu, C. M. Tsai, H. Y. Lin, Y. L. Wang, B. J. Hwang and C. C. Chen, Nano Energy, 2015, 11, 631 CrossRef CAS
.
- V. Mazumder, M. Chi, K. L. More and S. Sun, J. Am. Chem. Soc., 2010, 132, 7848 CrossRef CAS PubMed
.
- J. Zheng, R. Fu, T. Tian, X. Wang and J. Ma, Int. J. Hydrogen Energy, 2012, 37, 12994 CrossRef CAS
.
- D. S. Yang, M. S. Kim, M. Y. Song and J. S. Yu, Int. J. Hydrogen Energy, 2012, 37, 13681 CrossRef CAS
.
- J. T. Hwang and J. S. Chung, Electrochim. Acta, 1993, 38, 2715 CrossRef CAS
.
- W. Yuan, K. Scott and H. Cheng, J. Power Sources, 2006, 163, 323 CrossRef CAS
.
- A. N. Valisi, T. Maiyalagan, L. Khotseng, V. Linkov and S. Pasupathi, Electrocatalysis, 2012, 3, 108 CrossRef CAS
.
- K. Scott, W. Yuan and H. Cheng, J. Appl. Electrochem., 2007, 37, 21 CrossRef CAS
.
- Y. Gong, Y. D. Yeboah, S. N. Lvov, V. Balashov and Z. Wang, J. Electrochem. Soc., 2007, 154, 8560 CrossRef
.
- A. R. Malheiro, J. Perez and H. M. Villullas, J. Electrochem. Soc., 2009, 156, B51 CrossRef CAS
.
- A. R. Malheiro, J. Perez and H. M. Villullas, J. Power Sources, 2010, 195, 3111 CrossRef CAS
.
- A. Stassi, C. D'Urso, V. Baglio, A. Di Blasi, V. Antonucci, A. S. Aricò, A. M. Castro Luna, A. Bonesi and W. F. Triaca, J. Appl. Electrochem., 2006, 36, 1143 CrossRef CAS
.
- K. Shimizu, I. F. Cheng and C. M. Wai, Electrochem. Commun., 2009, 11, 691 CrossRef CAS
.
- X. Li, L. An, X. Wang, F. Li, R. Zou and D. Xia, J. Mater. Chem., 2012, 22, 6047 RSC
.
- Z. Sun and A. C. Tseung, Electrochem. Solid-State Lett., 2000, 3, 413 CrossRef CAS
.
- S. M. Foiles, M. I. Baskes and M. S. Daw, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 7983 CrossRef CAS
.
- S. Kang, S. Shi, Z. Jia, G. B. Thompson, D. E. Nikles, J. W. Harrell, D. Li, N. Poudyal, V. Nandwana and J. P. Liu, J. Appl. Phys., 2007, 101, 09J113 Search PubMed
.
- M. S. Seehral, V. Singh, P. Dutta, S. Neeleshwar, Y. Y. Chen, C. L. Chen, S. W. Chou and C. C. Chen, J. Phys. D: Appl. Phys., 2010, 43, 145002 CrossRef
.
- E. Antolini, T. Lopes and E. R. Gonzalez, J. Alloys Compd., 2008, 461, 253 CrossRef CAS
.
- N. M. Markovic and P. N. Ross, Surf. Sci. Rep., 2002, 45, 121 CrossRef
.
- H. A. Gasteiger, N. M. Markovic, P. N. Ross and E. J. Cairns, Electrochim. Acta, 1994, 39, 1825 CrossRef CAS
.
- H. Wang, J. Liang, L. Zhu, F. Peng, H. Yu and J. Yang, Fuel Cells, 2010, 10, 99 CAS
.
- Z. Wei, H. Guo and Z. Tang, J. Power Sources, 1996, 62, 233 CrossRef CAS
.
- J. Greeley and J. K. Nørskov, Electrochim. Acta, 2007, 52, 5829 CrossRef CAS
.
- A. Bonakdarpour, J. Wenzel, D. A. Stevens, S. Sheng, T. L. Monchesky, R. Lobel, R. T. Atanasoski, A. K. Schmoeckel, G. D. Vernstrom, M. K. Debe and J. R. Dahn, J. Electrochem. Soc., 2005, 152, A61 CrossRef CAS
.
- H. R. Colón-Mercado and B. N. Popov, J. Power Sources, 2006, 155, 253 CrossRef
.
- R. L. Borup, J. R. Davey, F. H. Garzon, D. L. Wood and M. A. Inbody, J. Power Sources, 2006, 163, 76 CrossRef CAS
.
- M. Watanabe, K. Tsurumi, T. Mizukami, T. Nakamura and P. Stoehart, J. Electrochem. Soc., 1994, 141, 2659 CrossRef CAS
.
- A. R. Malheiro, J. Perez and H. M. Villullas, J. Power Sources, 2010, 195, 7255 CrossRef CAS
.
- L. J. Wan, T. Moriyama, M. Ito, H. Uchida and M. Watanabe, Chem. Commun., 2002, 58 RSC
.
- A. K. Shukla, R. K. Raman, N. A. Choudhury, K. R. Priolkar, P. R. Sarode, S. Emura and R. Kumashiro, J. Electroanal. Chem., 2004, 563, 181 CrossRef CAS
.
- T. Itoh, M. Uebayashi, K. Tohji and B. Jeyadevan, Electrochemistry, 2010, 78, 157 CrossRef CAS
.
- Y. Hoshi, T. Yoshida, A. Nishikata and T. Tsuru, Electrochim. Acta, 2011, 56, 5302 CrossRef CAS
.
- Y. Hoshi, E. Tada, A. Nishikata and T. Tsuru, Electrochim. Acta, 2012, 85, 268 CrossRef CAS
.
- L. Xiong and A. Manthiram, J. Electrochem. Soc., 2005, 52, A697 CrossRef
.
- Q. Li, G. Wu, D. Su, H. Lv, S. Zhang, W. Zhu, A. Casimir, H. Zhu, A. Mendoza-Garcia and S. Sun, Nano Lett., 2015, 15, 2468 CrossRef CAS PubMed
.
- J. Zeng, S. Liao, J. Y. Lee and Z. Liang, Int. J. Hydrogen Energy, 2010, 35, 942 CrossRef CAS
.
- J. Kim, Y. Lee and S. Sun, J. Am. Chem. Soc., 2010, 132, 4996 CrossRef CAS PubMed
.
- S. Zhang, X. Zhang, G. Jiang, H. Zhu, S. Guo, D. Su, G. Lu and S. Sun, J. Am. Chem. Soc., 2014, 136, 7734 CrossRef CAS PubMed
.
- E. Antolini, Appl. Catal., B, 2016, 181, 298 CrossRef CAS
.
- J. Lee, J. M. Yoo, Y. Ye, Y. Mun, S. Lee, O. H. Kim, H. W. Rhee, H. I. Lee, Y. E. Sung and J. Lee, Adv. Energy Mater., 2015, 5, 1402093 Search PubMed
.
- L. Gan, R. Yu, J. Luo, Z. Cheng and J. Zhu, J. Phys. Chem. Lett., 2012, 3, 934 CrossRef CAS PubMed
.
- J. Cho, W. Roh, D.-K. Kim, J.-B. Yoon, J.-H. Choy and H. Kim, J. Chem. Soc., Faraday Trans., 1998, 94, 2835 RSC
.
- W. Roh, J. Cho and H. Kim, J. Appl. Electrochem., 1996, 26, 623 CrossRef CAS
.
- J. Shim, D.-Y. Yoo and J.-S. Lee, Electrochim. Acta, 2000, 45, 1943 CrossRef CAS
.
- B. Arumugam, T. Tamaki and T. Yamaguchi, ACS Appl. Mater. Interfaces, 2015, 7, 16311 CAS
.
- S. J. Hwang, S. J. Yoo, S. Jang, T.-H. Lim, S. A. Hong and S.-K. Kim, J. Phys. Chem. C, 2011, 115, 2483 CAS
.
- T. Tamaki, A. Minagawa, B. Arumugam, B. A. Kakade and T. Yamaguchi, J. Power Sources, 2014, 271, 346 CrossRef CAS
.
- B. Arumugam, B. A. Kakade, T. Tamaki, M. Arao, H. Imai and T. Yamaguchi, RSC Adv., 2014, 4, 27510 RSC
.
- K. Mohanraju and L. Cindrella, RSC Adv., 2014, 4, 11939 RSC
.
- M. T. Nguyen, R. H. Wakabayashi, M. Yang, H. D. Abruña and F. J. DiSalvo, J. Power Sources, 2015, 280, 459 CrossRef CAS
.
- J. Luo, L. Wang, D. Mott, P. N. Njoki, N. Kariuki, C.-J. Zhong and T. He, J. Mater. Chem., 2006, 16, 1665 RSC
.
- C.-J. Zhong, J. Luo, P. N. Njoki, D. Mott, B. Wanjala, R. Loukrakpam, S. Lim, L. Wang, B. Fang and Z. Xu, Energy Environ. Sci., 2008, 1, 454 CAS
.
- B. Fang, B. N. Wanjala, J. Yin, R. Loukrakpam, J. Luo, X. Hu, J. Last and C.-J. Zhong, Int. J. Hydrogen Energy, 2012, 37, 4627 CrossRef CAS
.
- B. Li and S. H. Chan, Int. J. Hydrogen Energy, 2013, 38, 3338 CrossRef CAS
.
- B. Wanjala, B. Fang, J. Luo, Y. Chen, J. Yin, M. Engelhard, R. Loukrakpam and C. J. Zhong, J. Am. Chem. Soc., 2011, 133, 12714 CrossRef CAS PubMed
.
- S.-W. Chou, J.-J. Shyue, C.-H. Chien, C.-C. Chen, Y.-Y. Chen and P.-T. Chou, Chem. Mater., 2012, 24, 2527 CrossRef CAS
.
- C.-J. Zhong, J. Luo, B. Fang, B. N. Wanjala, P. N. Njoki, R. Loukrakpam and J. Yin, Nanotechnology, 2010, 21, 062001 CrossRef PubMed
.
- J. Luo, N. Kariuki, L. Han, L. Wang, C.-J. Zhong and T. He, Electrochim. Acta, 2006, 51, 4821 CrossRef CAS
.
- B. Fang, J. Luo, P. N. Njoki, R. Loukrakpam, D. Mott, B. Wanjala, X. Hu and C.-J. Zhong, Electrochem. Commun., 2009, 11, 1139 CrossRef CAS
.
- B. Fang, J. Luo, P. N. Njoki, R. Loukrakpam, B. Wanjala, J. Hong, J. Yin, X. Hu, J. Last and C.-J. Zhong, Electrochim. Acta, 2010, 55, 8230 CrossRef CAS
.
- B. Fang, J. Luo, Y. Chen, B. N. Wanjala, R. Loukrakpam, J. Hong, J. Yin, X. Hu, P. Hu and C.-J. Zhong, ChemCatChem, 2011, 3, 583 CrossRef CAS
.
- M. Shahmiri, S. Murphy and D. J. Vaughan, Mineral. Mag., 1985, 49, 547 CAS
.
- V. S. Bagotzsky, Y. B. Vassiliev and O. A. Khazova, J. Electroanal. Chem., 1977, 81, 229 CrossRef
.
- R. Parsons and T. VanderNoot, J. Electroanal. Chem., 1988, 257, 9 CrossRef CAS
.
- T. Iwasita, Electrochim. Acta, 2002, 47, 3663 CrossRef CAS
.
- C. Coutanceau, S. Brimaud, C. Lamy, J.-M. Léger, L. Dubau, S. Rousseau and F. Vigier, Electrochim. Acta, 2008, 53, 6865 CrossRef CAS
.
- O. A. Petrii, J. Solid State Electrochem., 2008, 12, 609 CrossRef CAS
.
- N. M. Markovic, H. A. Gasteiger, P. N. Ross, X. Jiang, I. Villegas and M. J. Weaver, Electrochim. Acta, 1995, 40, 91 CrossRef CAS
.
- M. E. Tague, J. M. Gregoire, A. Legard, E. Smith, D. Dale, R. Hennig, F. J. DiSalvo, R. B. van Dover and H. D. Abruña, J. Electrochem. Soc., 2012, 159, F880 CrossRef CAS
.
- C.-T. Hsieh and J.-Y. Lin, J. Power Sources, 2009, 188, 347 CrossRef CAS
.
- E. Lee, S. Kim, J.-H. Jang, H.-U. Park, M. A. Matin, Y.-T. Kim and Y.-U. Kwon, J. Power Sources, 2015, 294, 75 CrossRef CAS
.
- S. Liang, F. Wang, Z. Zhang, Y. Li, Y. Cai, J. Ren and X. Jiang, RSC Adv., 2015, 5, 48569 RSC
.
- K. R. Lee, M. K. Jeon and S. I. Woo, Appl. Catal., B, 2009, 91, 428 CrossRef CAS
.
- M. Wen, H. Qi, W. Zhao, J. Chen, L. Li and Q. Wu, Colloids Surf., A, 2008, 312, 73 CrossRef CAS
.
- J. R. Rodriguez, R. M. Félix, E. A. Reynoso, Y. Gochi-Ponce, Y. Verde Gómez, S. Fuentes Moyado and G. Alonso-Núñez, J. Energy Chem., 2014, 23, 483 CrossRef
.
- J. Xu, K. Hua, G. Sun, C. Wang, X. Lv and Y. Wang, Electrochem. Commun., 2006, 8, 982 CrossRef CAS
.
- X. Ma, L. Luo, L. Zhu, L. Yu, L. Sheng, K. An, Y. Ando and X. Zhao, J. Power Sources, 2013, 241, 274 CrossRef CAS
.
- H. Zhao, L. Li, J. Yang, Y. Zhang and H. Li, Electrochem. Commun., 2008, 10, 876 CrossRef CAS
.
- J. Guo, Y. Sun, X. Zhang, L. Tang and H. Liu, J. Alloys Compd., 2014, 604, 286 CrossRef CAS
.
- N. K. Sahu, A. Prakash and D. Bahadur, Dalton Trans., 2014, 43, 4892 RSC
.
- M. E. Scofield, C. Koenigsmann, L. Wang, H. Liu and S. S. Wong, Energy Environ. Sci., 2015, 8, 350 CAS
.
- B. Luo, X. Yan, J. Chen, S. Xu and Q. Xue, Int. J. Hydrogen Energy, 2013, 38, 13011 CrossRef CAS
.
- C. X. Xu, Q. Li, Y. Q. Liu, J. P. Wang and H. R. Geng, Langmuir, 2012, 28, 1886 CrossRef CAS PubMed
.
- S. Liang, F. Wang, Z. Zhang, Y. Li, Y. Cai, J. Ren and X. Jiang, RSC Adv., 2015, 5, 48569 RSC
.
- Z. Ji, G. Zhu, X. Shen, H. Zhou, C. Wu and M. Wang, New J. Chem., 2012, 36, 1774 RSC
.
- Y. Wang, W.-P. Liao and Z.-H. Suo, J. Mol. Catal., 2013, 27, 356 CAS
.
- J. Zeng, Z. Zhao, J. Y. Lee, P. K. Shen and S. Song, Electrochim. Acta, 2007, 52, 3673 CrossRef CAS
.
- Q. Lv, Y. Xiao, M. Yin, J. Ge, W. Xing and C. Liu, Electrochim. Acta, 2014, 139, 61 CrossRef CAS
.
- J. Wu, J. Zhu, M. Zhou, Y. Hou and S. Gao, CrystEngComm, 2012, 14, 7572 RSC
.
- Y.-T. Liu, Q.-B. Yuan, D.-H. Duan, Z.-L. Zhang, X.-G. Hao, G.-Q. Wei and S.-B. Liu, J. Power Sources, 2013, 243, 622 CrossRef CAS
.
- N. M. Sánchez-Padilla, S. M. Montemayor, L. A. Torres and F. J. Rodríguez Varela, Int. J. Hydrogen Energy, 2013, 38, 12681 CrossRef
.
- P. Strasser, Q. Fan, M. Devenney, W. H. Weinberg, P. Liu and J. K. Nørskov, J. Phys. Chem. B, 2003, 107, 11013 CrossRef CAS
.
- M. K. Jeon, K. R. Lee, H. Daimon, A. Nakahara and S. I. Woo, Catal. Today, 2008, 132, 123 CrossRef CAS
.
- T. Huang, J. Liu, R. Li, W. Cai and A. Yu, Electrochem. Commun., 2009, 11, 643 CrossRef CAS
.
- M. K. Jeon, J. Y. Won, K. R. Lee and S. I. Woo, Electrochem. Commun., 2007, 9, 2163 CrossRef CAS
.
- T. Kim, K. Kobayashi, T. Take and M. Nagai, J. Oleo Sci., 2012, 61, 127 CrossRef CAS PubMed
.
- D.-Y. Wang, C.-H. Chen, H.-C. Yen, Y.-L. Lin, P.-Y. Huang, B.-J. Hwang and C.-C. Chen, J. Am. Chem. Soc., 2007, 129, 1538 CrossRef CAS PubMed
.
- D.-Y. Wang, H.-L. Chou, Y.-C. Lin, F.-J. Lai, C.-H. Chen, J.-F. Lee, B.-J. Hwang and C.-C. Chen, J. Am. Chem. Soc., 2012, 134, 10011 CrossRef CAS PubMed
.
- Z. Cai, Y. Kuang, X. Qi, P. Wang, Y. Zhang, Z. Zhang and X. Sun, J. Mater. Chem. A, 2014, 3, 1182 Search PubMed
.
- E. R. Gonzalez, E. A. Ticianelli and E. Antolini, in Catalysis in electrochemistry: from fundamental aspects to strategies for fuel cell development, ed. E. Santos and W. Schmickler, Wiley-VCH, 1st edn, 2011, pp. 453–485 Search PubMed
.
- S. Guo, S. Zhang, X. Sun and S. Sun, J. Am. Chem. Soc., 2011, 133, 15354 CrossRef CAS PubMed
.
- S. S. Kim, C. Kim and H. Lee, Top. Catal., 2010, 53, 686 CrossRef CAS
.
- X. Zhang, B. Zhang, D. Liu and J. Qiao, Electrochim. Acta, 2015, 177, 93 CrossRef CAS
.
- E. Antolini and E. R. Gonzalez, Catal. Today, 2011, 160, 28 CrossRef CAS
.
- J. Wang, S. Chen, P. Wang, R. Huang, M. Li and S. Sun, CIESC J., 2010, 61, 101 CAS
.
- L. Dong, H. Dong and J. Bai, 220th ECS Meeting Abstract #1164, The Electrochemical Society, 2011 Search PubMed
.
- H. Kivrak, N. C. Demir and Ö. Şahin, Selcuk University Journal of Engineering, Science and Technology, 2013, 1, 19 Search PubMed
.
- P. C. M. Gonzalez, S. M. Montemayor, D. M. Acosta, Y. Verde-Gomez, B. Escobar and F. J. R. Varela, J. New Mater. Electrochem. Syst., 2014, 17, 67 CAS
.
- T. S. Almeida, A. R. van Wassen, R. B. van Dover, A. R. de Andrade and H. D. Abruña, J. Power Sources, 2015, 284, 623 CrossRef CAS
.
- M. Ammam, L.E. Prest, A. D. Pauric and E. B. Easton, J. Electrochem. Soc., 2012, 159, B195 CrossRef CAS
.
- M. Ammam and E. B. Easton, J. Electrochem. Soc., 2012, 159, B635 CrossRef CAS
.
- M. Ammam and E. B. Easton, J. Power Sources, 2012, 215, 188 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |



