Highly specific determination of gentamicin by induced collapse of Au–lipid capsules†
Abstract
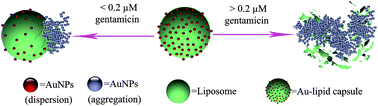
Residues of gentamicin in food pose a threat to human health. Determining gentamicin residues relies largely on adequate analytical techniques. Herein, a simple and highly specific colorimetric method for effective detection of this aminoglycoside antibiotic in milk based on gentamicin-induced collapse of an Au–lipid capsule is first proposed. The strong interaction between gentamicin and phosphatidylcholine resulted in the collapse of Au–lipid capsule and consequently, the color of AuNPs changed from wine red to blue. The concentration of gentamicin could be determined with the naked eye or a UV-vis spectrometer. The results showed that the absorption ratio (A664/A531) was liner with the gentamicin concentration in the 0–0.2 μM range with a linear 0.99 correlation coefficient. The detection limit was 7.4 nM. The coexisting substances, including L-arginine, guanidine hydrochloride, Tween-20, ammonium hydroxide, sodium chloride, potassium chloride, calcium chloride, glucose, and other common antibiotics such as streptomycin, amikacin, kanamycin, chloramphenicol, tetracycline, ampicillin and carbenicillin, did not interfere with the gentamicin determination with this method. Furthermore, the established method was successfully applied for the qualitative and quantitative analysis of gentamicin in pretreated milk products.

 Please wait while we load your content...
Please wait while we load your content...