Direct label-free detection of Rotavirus using a hydrogel based nanoporous photonic crystal†
Bohee Maenga,
Youngkyu Parkb and
Jungyul Park*ac
aDepartment of Mechanical Engineering, Sogang University, Sinsu-dong, Mapo-gu, Seoul, 121-742, Korea. E-mail: sortpark@sogang.ac.k; Fax: +82-2-712-0799; Tel: +82-2-705-8642
bAgency for Defense Development, Yuseong, P.O. Box 35, Daejeon, 305-600, Korea
cInterdisciplinary Program of Integrated Biotechnology, Sogang University, Sinsu-dong, Mapo-gu, Seoul, 121-742, Korea
First published on 5th January 2016
Abstract
To date, there is no commercial point-of-care test (POCT) for intact virus detection. A POCT should show high sensitivity and have proper structure through simple and cost effective fabrication; moreover it should not require a complex and bulky instrument for measurement. In this study, a direct label-free biosensor based on 3D photonic crystal (PC) structures for Rotavirus has been demonstrated, which can be developed for POCT. The proposed sensor can detect the target with viral loading from 6.35 μg ml−1 to 1.27 mg ml−1 without any sample pretreatment. Sensitivity and selectivity performance are analyzed quantitatively by measuring peak wavelength value (PWV) and comparing the performance using an ELISA assay. In order to facilitate the target virus accessibility to inner structures in the sensor, the hydrogel based inverse opal structure is etched by O2 plasma and the connection window between nanopores becomes enlarged. This process is critical for enhancing virus sensing performance. Our PWV shift range is larger than of the shift in a 1D photonic crystal based virus sensor because the larger surface area in 3D inverse opal structures is realized. These results show that the proposed method is useful for developing a direct and easy-to-use virus detection kit in the form of a POCT in the near future.
1. Introduction
Photonic crystals (PC) are increasingly attracting attention due to their unique structure colors and high potential in various fields.1,2 PC possess a periodic arrangement of dielectric material and due to this periodicity, may exhibit a specific color or peak wavelength value (PWV).3 This color can be changed by physiochemical changes induced from an external stimulus. Therefore, PC can be used as a colorimetric sensor transforming environmental changes into visual color changes and can be realized in a low-cost and battery-free sensor.4 To date, various types of PC sensors have been reported for measuring solvents, vapors, temperature, ionic strength, pH, biomolecules, and mechanical force.1,5–9 In 2004, Sharma et al. developed a new photonic crystal sensor useful as a point-of-care clinical sensor for the detection and quantification of creatinine, which is an important small molecule marker of renal dysfunction.10 In 2011 Arunbabu et al. reported a PC hydrogel for the sensing of the highly toxic mercury ion (Hg2+), which can cause numerous disorders, including sensory and neurological damage in the human body.11 Recently, in 2015, a three-dimensional (3D) PC sensor that can selectively detect organic vapors, which can cause damage to both the environment and human health, through visual color changes for on-line and real-time monitoring was developed by Zhang et al.12 The same year, the neurotransmitter acetylcholine could be detected for diagnostic analysis and evaluation of treatment effects by combining an enzyme hydrogel with a 3D PC hydrogel layer that responds to ionic strength and pH changes.13 For detecting harmful volatile organic compounds such as alcohols, formaldehyde, toluene and acetone, Wang et al. studied cellulose film with a 3D colloidal array embedded inside.14PC sensors can be divided into two types: fluorescence-based detectors and label-free detectors.15 In fluorescence-based detection, PC play the role of a Bragg mirror to enhance the excitation signal, which matches the PWV; therefore, the sensitivity of sensors can be improved dramatically. Label-free biosensors detect biomolecules through changes of the PWV when biological molecules, such as proteins, viruses or cells, bind to the PC surface.3,15 This label-free method is more attractive than fluorescence labeling for many reasons: (1) portable point-of-care testing (POCT) is possible without the time-consuming and expensive fluorescence labeling step for the targets and the heavy and expensive fluorescent equipment required. (2) Quantitative measurement of molecule interaction is allowable. (3) Finally, this PC biosensor can be used as a colorimetric biosensor that can identify the quantity of biomolecule binding levels.8
Rotavirus is a viral agent of gastroenteritis in animals and humans and is distributed worldwide.16,17 It is one of the common enteric agents and mortality associated with its infection can be very significant for infants and children under 5 year, especially in developing countries.3 For detection of the virus, in earlier days, electron microscopy was used by confirming their morphology.18 Currently, there are many techniques and publications for virus diagnosis. ELISA and RT-PCR are the usual and classic diagnostic methods,16,18,19 and surface plasmon resonance (SPR) and quartz crystal microbalance (QCM) are also studied for detection of intact virus particles.3,16 Although SPR and QCM offer a label-free biosensing with high sensitivity, their expensive and complex instrument is a bottleneck for development as a POCT. Virus detection through POCT has been technically challenging and no POCT platform for viral load has been available commercially.20
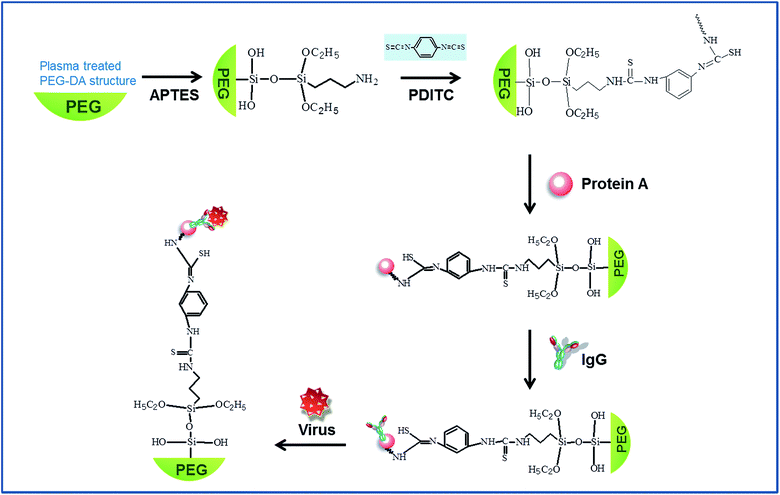
In this study, we present a direct label-free detection method for Rotavirus using a 3D PC biosensor, as shown in Fig. 1. The 3D inverse opal sensor that we proposed in this study for detection of the virus is essentially made up of a hydrogel backbone. Because these hydrogels are known to show high capacity for biomolecule immobilization, greater probability of interacting with the target molecules and high LOD, S/N, and sensitivity, they have been commercialized by some companies in microarrays.21–24 Previously, highly sensitive biosensing for biomolecular screening has been investigated using 3D nanoporous hydrogel by several research groups, including us.21,25,26 Another advantage is that a 3D inverse opal structure has larger voids that can accommodate more intensive variation in their refractive index compared to an opal structure.15,27 In addition, the presence of nanopores can not only provide greater surface area and more interaction sites, but also offer easier access for the analytes to the recognition sites.15,27 Especially, in order to facilitate the permeability of target analytes into nanoporous structures in the sensor, the hydrogel backbones in the inverse opal structure were treated with reactive ion etching (RIE). This resulted in enlarging the area of the interconnection windows between nanopores, and the analytes could access deeply into the sensor through the windows, and it critically affects the detection performance. For pathogen specificity, the surface of inverse opal structures is modified with protein A and specific monoclonal Rotavirus antibody. The proposed system can quantitatively detect a target virus load from 6.35 μg ml−1 to 1.27 mg ml−1 by measuring the PWV shift without any pretreatment. The specific capturing on the functionalized surfaces is confirmed by fluorescence and is visualized with gold nanoparticle labelling using scanning electron microscopy (SEM). Finally, the virus detection limit is compared with a commercial ELISA kit.
2. Materials and method
2.1 Fabrication of sensor structures
The fabrication process of inverse opal structures is similar to our previous work.8 More details are shown in Fig. S1 of the ESI.† First, colloidal crystal based silica (SiO2) nanoparticles (0.351 μm, microparticles GmbH, Germany) were dropped onto a glass slide and it was covered with a piece of cover glass for self-assembly. After full evaporation, the silica beads were assembled to the shape of the face-centered cubic (FCC) structure. The cover glass was removed and the assembled structure was immersed into ethylene glycol diacrylate (PEG-DA) solution for 5 min. Then, the assembled structure was covered with a polyethylene terephthalate (PET) film that had been modified with urethane groups to increase adhesion to the acrylate-containing monomer. PEG-DA solution was prepared from 99 wt% PEG-DA (Sigma Aldrich) and 1 wt% of the water soluble photoinitiator 2-hydroxy-2-methylpropiophenone (Sigma Aldrich). To minimize the swelling effect of the PEG-DA backbone, a low molecular rate (MN258) PEG-DA material was used. This sample was exposed to UV (250–400 nm) for a few tens of seconds at 90 mW cm−2. An inverse opal structure was obtained by wet etching the silica beads in a buffer oxide etchant. The fabricated inverse opal PC structure was etched by O2 plasma in order to ensure an ideal condition for virus impregnation. It was conducted under the condition of 100 W power for 30 s.2.2 Surface functionalization on a sensor surface and PWV measurement
For a specific pathogen, the PEG-DA surface of the fabricated structures was functionalized with the protocol as follows (Fig. 2): the sample was first immersed in a 10% solution of (3-aminopropyl)triethoxysilane (APTES, Sigma Aldrich) in ethanol for 2 h. Then, it was washed with ethanol. Furthermore, 1,4-phenylene diisothiocyanate (PDITC, Tokyo Chemical Industry, Japan) in N,N-dimethylformamide (DMF, Sigma Aldrich) was reacted with aminopropyl surface for 2 h at 25 °C in darkness. Then, a washing step with DMF and ethanol was followed and 500 μg ml−1 protein A in PBS (pH 7.2) was incubated on the structure surface for 2 h at 37 °C. Protein A immobilized on the surface was functionalized with a capture antibody (anti-Rotavirus IgG, Fitzgerald) by incubating in the antibody solution for 2 h at 37 °C. It was washed with PBS and then 5% bovine serum albumin (BSA, Sigma Aldrich) was used for the blocking step to prevent nonspecific binding. The PWV shift of each surface functionalization step and virus detection was measured by an optical spectrum analyzer (Ocean optics HR4000, USA), which was plugged into the bifurcated optic fiber. Before measuring the PWV shift due to each chemical step and virus detection, the samples should be sufficiently dried.2.3 Preparation of a Rotavirus sample and verification process for virus detection
In this study, we used commercially available virus antigen (Rotavirus antigen, strain SA11, Jena Bioscience). The initial concentration of 1.27 mg ml−1 of virus sample was diluted with PBS into a range of five concentrations, including the original sample (10, 100, 200, 500 fold, respectively) from 2.54 μg ml−1 to 127 μg ml−1. 20 μl of each virus sample was reacted on the functionalized sensor at the final step. As a negative control group, PBS is used. After 2 h reaction time at 37 °C, the PWV shift was measured.In order to verify that the proposed surface modification works properly for virus detection, after the reaction of the virus samples on the sensor surface, Rotavirus polyclonal goat antibody (Fitzgerald, USA) and FITC labeled anti-goat IgG (Fitzgerald, USA) were incubated. Each step was reacted for 2 h at 37 °C. Detected viruses on the sensor pores were identified by a fluorescent signal using an inverted fluorescence microscope (Olympus IX71). For checking the successful attachment of Rotavirus on the sensor surface, scanning electron microscopy (SEM, SIGMA, Carl Zeiss) was used and gold nanoparticles-tagged antibody was conjugated on the virus for easy observation.28,29 For immobilizing gold nanoparticles, 18 nm gold nanoparticle-labeled immunoglobulin antibody was reacted (abcam, UK). In order to prepare samples for SEM, the 3D PC sensors that finally reacted with virus or gold nanoparticles-tagged virus were rinsed with PBS and fixed with 1% glutaraldehyde (Sigma Aldrich) in PBS at 4 °C for 1 h. Washing with PBS was followed by 1% osmium tetroxide (Sigma Aldrich) in PBS at 4 °C for 15 min. The structure was then washed with DI water three times. Samples were dehydrated using a graded ethanol series (25%, 50%, 75%, 80%, 90%, and 100% diluted ethanol in DI water) and a drying step was conducted.
2.4 Sensor specificity for virus detection
Sensor specificity was demonstrated by comparing the PWV shift of diverse types of virus samples. Echo and Influenza A virus antigen were compared to the Rotavirus. Echo and Influenza A virus (Jena Bioscience, Germany) were diluted to a final concentration of 2.5 and 2.26 mg ml−1, respectively. These concentrations were almost twice higher than the initial concentration of Rotavirus. The surfaces of the 3D PC structures were functionalized as described above and finally they were reacted to each virus's antigens. The PWV shifts were measured from completely dried sensors.2.5 Commercial ELISA assay test
An enzyme linked immunosorbent assay (ELISA) specific for Rotavirus group A antigen was performed using a commercial ELISA kit (IVD Research, USA) as described in the manufacture's instruction. The same concentrations of Rotavirus antigen tested on 3D PC sensors were assessed using the ELISA test.3. Results and discussion
3.1 Analysis of PWV shift by surface modification and Rotavirus detection
Since PCs are highly sensitive to the refractive index of its constituents, the changes in refractive index due to the capture of biological species can be detected as shifts in the reflectance spectrum.30 The wavelength of the reflected light, PWV (λ), could be described by Bragg's equation as follows.1λ = 2d(neff2 − cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)1/2 θ)1/2 |
The effective refractive index of the constituent PC is expressed as neff, d is the distance of diffracting plane spacing, and θ is the Bragg angle of incidence of the light falling on the nanostructures.1
The surface modification reaction for Rotavirus detection on the fabricated sensors was analyzed by PWV shift with respect to each modification step, as shown Fig. 3. Each PWV shift represents the difference in PWV between the current and the previous surface modification step. After functionalization with protein A, a PWV shift of 5.19 ± 0.82 nm was measured. Then, after the captured antibodies were reacted sequentially, the difference in PWV from the previous step was measured to be 7.47 ± 0.66 nm. Although the blocking step is important for preventing non-specific binding, it was known that this step did not cause a statistically significant PWV shift compared to the antibody capture step.20 The PWV shift after the blocking step with 1% BSA was 1.05 ± 0.45 nm.
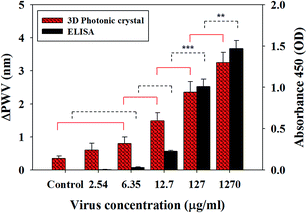
The performance of the virus sensor for detection of Rotavirus was also evaluated using the PWV shift. Various concentrations from the diluted virus solutions were tested to determine the sensitivity of the sensor. As a result, the PWV shift, according to virus concentration, showed a statistically significant difference compared to control samples (just PBS solution). The PWV shift at the highest concentration 1.27 mg ml−1 was almost 3.25 ± 0.31 nm. Shifts of 2.36 ± 0.32 nm and 1.48 ± 0.24 nm were measured after the reaction with 10 (127 μg ml−1) and 100 times (12.7 μg ml−1) diluted virus solution, respectively (Fig. 4, n = 5, p < 0.05). We also found that the PWV shift in the 200 times diluted solution (6.35 μg ml−1) was 0.8 ± 0.31 nm (n = 5, p < 0.05). It could be clearly distinguishable compared to the control group. However, as described in Fig. 4, there was no clear difference in PWV shift between the 2.54 μg ml−1 viral sample and the control group (n = 5, p > 0.05). From these data, it was verified that the proposed sensor was able to detect virus quantitatively, according to the change of the virus concentration, and its minimum resolution was 6.35 μg ml−1. Pineda et al. previously reported a 1D photonic crystal biosensor for detecting Rotavirus that has active viral infection.3 However, in this study, commercially available inactivated virus was used for safety issue, so it is difficult to make a focus-forming assay (FFA). This means it is not possible to convert our virus loading unit into FFU or copies per ml for direct comparison of our result with another group's results, but the function of inactivated virus is not different from real active samples except for the inactive treatment for safety. They have the same size, shape and specific antibody reactions as real samples, which are commonly used for validation of biosensors. Previously, Nakano et al. used commercially available inactivated Rotavirus for sensor detection31 and inactivated Influenza A virus also used for sensor study.32 Many literatures mentioned that our Rotavirus (strain SA11, Simian group A Rotavirus) is the same strain as the Human serotypes of group A Rotavirus, which is a major cause of gastroenteritis in young children throughout the world.16,19,33 When considering the PWV shift range, our PWV shift range (nanometer change) was much larger than the shift range in previous work (picometer range) because the larger surface area in 3D inverse opal structures was realized. This means more sensitive virus detection is possible using our system.
3.2 Comparison between PC sensor and ELISA
Since the virus used in this study is an inactive agent, it is difficult to compare the sensitivity performance directly using a focus-forming assay (FFA). Therefore, we carried out the ELISA assay for indirect comparison with a previous study.3 ELISA is a widely used virus detection method due to its high sensitivity.Fig. 4 shows the comparison of sensitivity between the proposed PC sensor and ELISA. The PC sensor shows a similar limit of detection compared with the ELISA assay. In the previous work,3 the sensing performance using 1D photonic crystal was also comparable with the commercially available ELISA kit. Using the ELISA assay, it was difficult to detect less than 6.35 μg ml−1 concentration virus samples. This value was similar to the minimum detection level of the PC sensor, as described above. The ELISA assay results showed that the absorbance dropped off remarkably below 127 μg ml−1 level of virus concentration. However, in the PC sensor, the PWV shift has shown linear variation according to the virus concentration decrease; i.e., the PWV shift range went down steadily with respect to decreased virus concentration. Therefore, it will be a more useful tool to analyze virus concentration quantitatively. We demonstrated that the PC sensor had sufficient sensitivity, which was comparable or superior to commercially available ELISA.
3.3 Specific detection of a 3D PC sensor
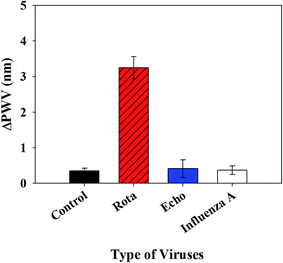
To confirm the specificity of the 3D PC sensor, we tested several different types of virus antigens other than Rotavirus. Echo and Influenza A virus antigens were additionally tested and the PWV shifts were compared with Rotavirus, the target of our PC sensor.Each 3D PC structure was functionalized with the same procedure that we established. The measured PWV shifts of each virus, including the control which was reacted with PBS buffer, are demonstrated in Fig. 5. In case of Echo and Influenza A virus, the PWV shifts were not distinguishable from the control group, which was reacted with no virus agents, just PBS solution, although the two viruses were exposed to the PC sensor with an almost twice higher concentration than Rotavirus. It was confirmed that the 3D PC sensor specifically detected Rotavirus, as we designed.
3.4 Visual analysis for 3D PC sensor
The specific binding for Rotavirus into the 3D PC structures was visually confirmed by observing the fluorescence intensity with a fluorescence microscope, as shown in Fig. 6, and using scanning electron microscopy (SEM) imaging, as shown in Fig. 7.In order to check the specific binding performance, the proposed biomolecule binding reaction was carried out on a glass, a bare PEG-DA flat surface and an inverse opal PC structure. First, the fluorescent signal from the sample that allows non-specific binding (has no capture antibody) was compared to the reference sample that was not treated at all. There was no difference in fluorescent signals between the two groups. However, a definite intensity change was observed when the sample for specific binding was compared with the sample for non-specific binding. Especially, there was a much higher signal from the 3D inverse opal structure than the signal from the 2D flat surface, such as the glass or PEG-DA substrate, as shown in Fig. 6(d). This supported that the 3D inverse opal structure could have virus detection ability superior to the 2D flat glass and the flat PEG-DA substrate, because the 3D inverse opal structure could allow enough interconnection sites for binding the virus sample. Moreover, with the flat PEG-DA and glass surface, higher intensity was observed in the flat PEG-DA, and it was proven that intrinsically more biomolecule immobilization and higher probability of interacting with the target can be realized on hydrogel than on glass.
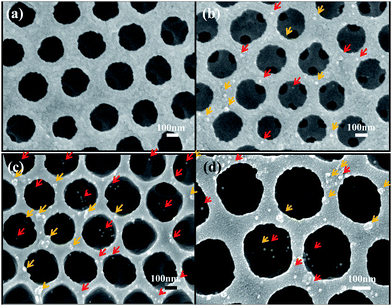
Metal nanoparticles tagged on viruses were used for clearer observation by SEM.28,29 Fig. 7 shows the surface of sensors after incubation with virus solution and tagging with 18 nm size gold nanoparticles conjugated with monoclonal antibody for 2 h. By using this method, it is possible to easily observe the detected virus particles on the inverse opal structure by finding the attached gold nanoparticles. It was well known that the wheel-like round shaped structure is the characteristic feature of the Rotavirus and its regular size is 50–80 nm in diameter.18 Herein, from the SEM image, we were able to identify the virus of about 50–70 nm size with round shape on the sensor surface (Fig. 7). Moreover, we found that the shiny gold particles tagged on the virus were scattered inside the pores from photonic crystal structures.
Finally, we compared the sensor performance upon RIE treatment that induces deeper penetration of the virus into internal structures. When the inverse opal structure was fabricated using colloidal particles, the connection windows between nanopores were narrow. It was not wide enough for viruses to pass through freely. To facilitate the target virus's access into inner structures, O2 plasma etching by RIE was implemented, and the size of the interconnection windows was evidently increased more than 100 nm, as shown in Fig. 7(c) and (d). The enhanced virus detection ability was verified definitely by comparing the SEM images between non-RIE treated and RIE treated samples. In the case of RIE treated sample (Fig. 7(c) and (d)), there were many gold particle-tagged viruses inside nanopores, but it was difficult to find the virus inside nanpores in the case of non-RIE treated sample (Fig. 7(b)). To determine the optimal etching condition, we compared the morphology in the nanopore structures depending on the reaction time. More detail is available in the ESI (Fig. S2†).
4. Conclusion
In this study, we demonstrated a label-free biosensor based on inverse opal PC structures for detecting intact Rotavirus. We successfully set up the surface modification process for virus sensing and confirmed this pathogen specific protocol by comparing PWV shifts using other virus agents and labelling secondary fluorescent antibody. The virus detection performance according to virus concentration was investigated by measuring the PWV shift and it was shown that the PWV shift indicated the quantitative concentration of the specific target virus agents. The sensitivity of our proposed system is compatible with or superior to the commercial ELISA kit. Capture of the target virus was confirmed by SEM with gold nanoparticle tagging and through SEM images. The RIE process allowing easier virus access into sensor structures was proved to be critical for enhancement of virus detection performance.This study provides direct and quantitative virus detection by a simple process without any pretreatment for the target virus and would be helpful in developing a direct and easy-to-use virus detection kit in the form of POCT in the near future.
Acknowledgements
This study was supported by the Basic Science Research Program (2012R1A1A2003473, 2013R1A1A2073271) and by the National Nuclear R&D Program (2012M2A8A4055325) through the National Research Foundation of Korea and also by the Basic Science Research Program through Agency for Defence Development.4. References
- H. Wang and K. Q. Zhang, Sensors, 2013, 13, 4192–4213 CrossRef CAS.
- Y. J. Zhao, Z. Y. Xie, H. C. Gu, C. Zhu and Z. Z. Gu, Chem. Soc. Rev., 2012, 41, 3297–3317 RSC.
- M. F. Pineda, L. Li-Ying Chan, T. Kuhlenschmidt, C. J. Choi, M. Kuhlenschmidt and B. T. Cunningham, IEEE Sens. J., 2009, 9, 470–477 CrossRef CAS.
- M. M. Hawkeye and M. J. Brett, Adv. Funct. Mater., 2011, 21, 3652–3658 CrossRef CAS.
- Y. J. Lee and P. V. Braun, Adv. Mater., 2003, 15, 563–566 CrossRef CAS.
- J. M. Weissman, H. B. Sunkara, A. S. Tse and S. A. Asher, Science, 1996, 274, 959–960 CrossRef CAS.
- J. H. Kim, J. H. Moon, S. Y. Lee and J. Park, Appl. Phys. Lett., 2010, 97, 103701–103703 Search PubMed.
- E. Choi, Y. Choi, Y. H. P. Nejad, K. Shin and J. Park, Sens. Actuators, B, 2013, 180, 107–113 CrossRef CAS.
- H. Fudouzi and T. Sawada, Langmuir, 2006, 22, 1365–1368 CrossRef CAS.
- A. C. Sharma, T. Jana, R. Kesavamoorthy, L. J. Shi, M. A. Virji, D. N. Finegold and S. A. Asher, J. Am. Chem. Soc., 2004, 126, 2971–2977 CrossRef CAS.
- D. Arunbabu, A. Sannigrahi and T. Jana, Soft Matter, 2011, 7, 2592–2599 RSC.
- Y. Q. Zhang, J. H. Qiu, R. R. Hu, P. Li, L. J. Gao, L. P. Heng, B. Z. Tang and L. Jiang, Phys. Chem. Chem. Phys., 2015, 17, 9651–9658 RSC.
- C. Fenzl, C. Genslein, A. Zopfl, A. J. Baeumner and T. Hirsch, J. Mater. Chem. B, 2015, 3, 2089–2095 RSC.
- F. Y. Wang, Z. G. Zhu, M. Xue, F. Xue, Q. H. Wang, Z. H. Meng, W. Lu, W. Chen, F. L. Qi and Z. Q. Yan, Sens. Actuators, B, 2015, 220, 222–226 CrossRef CAS.
- Y. Zhao, X. Zhao and Z. Gu, Adv. Funct. Mater., 2010, 20, 2970–2988 CrossRef CAS.
- J. D. Driskell, Y. Zhu, C. D. Kirkwood, Y. Zhao, R. A. Dluhy and R. A. Tripp, PLoS One, 2010, 5, e10222 Search PubMed.
- N. Guttman-Bass and R. Armon, Appl. Environ. Microbiol., 1983, 45, 850–855 CAS.
- E. J. Anderson and S. G. Weber, Lancet Infect. Dis., 2004, 4, 91–99 CrossRef.
- I. Gutierrez-Aguirre, A. Steyer, J. Boben, K. Gruden, M. Poljsak-Prijatelj and M. Ravnikar, J. Clin. Microbiol., 2008, 46, 2547–2554 CrossRef CAS.
- H. Shafiee, E. A. Lidstone, M. Jahangir, F. Inci, E. Hanhauser, T. J. Henrich, D. R. Kuritzkes, B. T. Cunningham and U. Demirci, Sci. Rep., 2014, 4, 4116 Search PubMed.
- Y. Zhao, X. Zhao, B. Tang, W. Xu and Z. Gu, Langmuir, 2010, 26, 6111–6114 CrossRef CAS.
- M. F. Ali, R. Kirby, A. P. Goodey, M. D. Rodriguez, A. D. Ellington, D. P. Neikirk and J. T. McDevitt, Anal. Chem., 2003, 75, 4732–4739 CrossRef CAS.
- B. D. Martin, C. M. Soto, C. Taitt and P. T. Charles, Biotechnol. Bioeng., 2008, 99, 1241–1249 CrossRef CAS.
- P. T. Charles, C. R. Taitt, E. R. Goldman, J. G. Rangasammy and D. A. Stenger, Langmuir, 2004, 20, 270–272 CrossRef CAS.
- Y. Lee, S. Park, S. W. Han, T. G. Lim and W. G. Koh, Biosens. Bioelectron., 2012, 35, 243–250 CrossRef CAS.
- C. Wang, C. Y. Lim, E. Choi, Y. Park and J. Park, Sens. Actuators, B, 2016, 223, 372–378 CrossRef CAS.
- W. P. Qian, Z. Z. Gu, A. Fujishima and O. Sato, Langmuir, 2002, 18, 4526–4529 CrossRef CAS.
- J. A. Padilla, F. Uno, M. Yamada, H. Namba and S. Nii, J. Electron Microsc., 1997, 46, 171–180 CrossRef CAS.
- O. E. Olorundare, O. Peyruchaud, R. M. Albrecht and D. F. Mosher, Blood, 2001, 98, 117–124 CrossRef CAS.
- Y. Guo, J. Y. Ye, C. Divin, B. Huang, T. P. Thomas, J. R. Baker Jr and T. B. Norris, Anal. Chem., 2010, 82, 5211–5218 CrossRef CAS PubMed.
- M. Nakano, R. Obara, D. Zhenhao and J. Suehiro, International Conference on Sensing Technology: Proceedings 7th Int. Conf. on Sensing Technology, New zealand, 03–05 Dec 2013, pp. 374–378 Search PubMed.
- L. Farris, N. Wu, W. Wang, L.-J. Clarizia, X. Wang and M. McDonald, Anal. Bioanal. Chem., 2010, 396, 667–674 CrossRef CAS.
- Y. Hoshino, R. W. Jones and A. Z. Kapikian, Arch. Virol., 1998, 143, 1233–1244 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21665f |
| This journal is © The Royal Society of Chemistry 2016 |