Nano-K2CO3: preparation, characterization and evaluation of reactive activities†
Jun-Zhang Li,
Shi-Ming Fan,
Xuan-Fei Sun and
Shouxin Liu*
State Key Laboratory Breeding Base, Hebei Laboratory of Molecular Chemistry for Drug Research, Hebei University of Science and Technology, 70 Yuhua East Road, Shijiazhuang 050018, China. E-mail: chlsx@hebust.edu.cn; Fax: +86-311-8863-2254; Tel: +86-311-8863-2254
First published on 18th December 2015
Abstract
A novel base, nano-K2CO3, was easily prepared by ultrafine wet milling. The surface properties and the reactive activities of nano-K2CO3 were characterized. It was found that such a base showed higher basicity than normal K2CO3 and could replace sodium (or potassium) alkoxide to carry out monoalkylation and oximation of active methylene compounds. The nano-K2CO3 could be regenarated and reused 10 times without loss of its reactive activity.
Introduction
Traditional strong bases such as sodium (or potassium) alkoxide have been used widely in the organic synthesis industry, but these bases have a number of shortcomings such as non-recovery, high cost, severe corrosion and more side reactions. K2CO3 is a good base because it is lowcost, noncorrosive and easy to separate from products in non-aqueous media. However, its low activity limits its efficiency in practical applications. Recently, nano-materials as catalysts have been receiving considerable attention because of their high specific surface area and special reactivities.1 Compared with traditional heterogeneous reaction, nanocatalyst-based heterogeneous reaction can efficiently improve activity and selectivity, as well as enable transformations of reaction substrate under much more gentle conditions and products isolation.2 Numerous nano-metals such as Pt, Pd, Mg, Cu, Ni, and their metal oxide have been widely applied in various organic reactions.3 Many nano-inorganic salts such as alkaline-earth metals carbonate (MCO3) have been reported,4 but so far there is just a little of reports on nano-alkali metal carbonates (M2CO3).5 We previously reported that the preparation of macronano-K2CO3 with average particle size of 266 nm.6 In this work, we developed a simple method of preparing nano-K2CO3 with average particle size of 64 nm and used it as a base to evaluate its properties through the monoalkylation and oximation of active methylene compounds.Results and discussion
Based on the ionic characteristics of K2CO3, nano-K2CO3 was prepared by ultrafine wet milling.7 The particle size of nano-K2CO3 strongly depends on solvent, with polar and protic being more suitable for the preparation of nano-K2CO3 (Table 1, entries 3 and 4) than nonpolar and aprotic (Table 1, entries 1 and 2). A possible reason may be that polar solvents have strong electrostatic interactions with the charge of nano-K2CO3 surface to retard the agglomeration and contribute to the dispersion of nanoparticles. The anion of nano-K2CO3 surface can be intensively solvated by protic solvent through the interaction of hydrogen bond to decrease the surface energy of nanoparticle. Given the properties of hydroxyl bond in the dispersion and stabilization of nano-K2CO3 particles, lauric acid was selected as an auxiliary to prepare nano-K2CO3 with smaller particle size. When 0.1% mol lauric acid was added to ethanol, K2CO3 particles obtained by milling obviously decreased (Table 1, entries 4 and 5). By controlling lauric acid with 0.2% mol of ethanol, 64 nm nano-K2CO3 with average particle size (Table 1, entry 6) and narrow particle size distribution was prepared (ESI; Fig. S1†). The SEM image of nano-K2CO3 is shown in Fig. 1. Nano-K2CO3 was easily agglomerated when exposed to air. Hence, it was stored in the solvents of ethanol/0.2% lauric acid and directly used for the reaction.| Entry | Solventa | Timeb (h) | Average sizec (nm) |
|---|---|---|---|
a Solvent/potassium carbonate (molar ratio = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).b Average size does not decrease with prolonged grinding time.c Average size was measured using a laser particle size analyzer.d Molar percentage of nano-K2CO3. 1).b Average size does not decrease with prolonged grinding time.c Average size was measured using a laser particle size analyzer.d Molar percentage of nano-K2CO3. |
|||
| 1 | Toluene | 8 | 893 |
| 2 | Acetonitrile | 10 | 775 |
| 3 | 1-Butanol | 10 | 581 |
| 4 | Ethanol | 8 | 266 |
| 5 | Ethanol/0.1% lauric acidd | 8 | 112 |
| 6 | Ethanol/0.2% lauric acid | 8 | 64 |
| 7 | Ethanol/0.3% lauric acid | 8 | 64 |
The basic strength and relative amount of surface basic sites on nano-K2CO3 were studied by CO2-TPD (Fig. 2). CO2-TPD peak area showed that the total capacity of CO2 adsorption of nano-K2CO3 was four times higher than that of normal K2CO3 (Table 2). Obviously, the adsorption of nano-K2CO3 could be ascribed to its high specific surface area and characteristic surface property. For normal K2CO3, the desorption peak α located at around 60–160 °C interval was observed, which may be due to physisorption. However, two desorption peaks were observed for nano-K2CO3. Except for peak α, the other peak β located at around 170–220 °C was distinct, which could be due to the characteristic basic site caused by the exposed anion of nano-K2CO3 surface. This finding suggested that a new type of adsorption site with stronger basicity existed on nano-K2CO3 surface. Given the greater number of characteristic basic sites exposed on the surface, nano-K2CO3 activity was greatly improved.
To examine the chemical property of nano-K2CO3, monoalkylation and oximation of active methylene compounds were performed. The monoalkylation of active methylene compounds with alkyl halides is a very useful reaction for prolonging the carbon chain.8 The oximation of active methylene compounds with nitrous esters is a very important reaction for the preparation of oximes, which are particularly useful precursors for the synthesis of α-amino acids9 and heterocyclic compounds.10 Traditionally, both of these reactions often require a strong basic medium, such as a solution of sodium alkoxide/alcohol. However, sodium ethoxide and sodium hydride is more expensive and lacks the selectivity for the alkylation of active methylene compounds, easily producing a mixture of mono- and di-alkylation products. In particular, a amount large of wastewater was produced during work-up in chemical industry. In this work, nano-K2CO3 was used as a base in nonaqueous media to replace to sodium alkoxide or sodium hydride and carry out both reactions. Both heterogenous reaction systems of monoalkylation and oximation of active methylene compounds were successfully constructed.
The alkylation of diethyl malonate with n-propyl bromide was initially examined. When 1.3 equiv. of nano-K2CO3 was used as base, the product was achieved in 85.2% yield at 65 °C (Table 3, entry 1). However, the reaction occurred in the presence of a large excess of normal potassium carbonate (3.5 equiv.), the product was obtained in 65% yield after 8 h at 75 °C.
| Entry | W1 | W2 | R–X | T (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Yield of isolated product. | ||||||
| 1 | CO2Et | CO2Et | n-PrBr | 65 | 8 | 85.2 |
| 2 | CO2Et | CO2Et | EtBr | 65 | 8 | 87.4 |
| 3 | CO2Et | CO2Et | n-BuBr | 65 | 8 | 82.6 |
| 4 | CO2Et | CO2Et | PhCH2Cl | 65 | 8 | 89.3 |
| 5 | CO2Et | CO2Et | Br(CH2)3Cl | 65 | 8 | 87.5 |
| 6 | CO2Et | CO2Et | Br(CH2)5Cl | 65 | 8 | 85.8 |
| 7 | Ph | CN | BrCH2CO2Et | 75 | 15 | 62.4 |
| 8 | Ph | CN | EtBr | 75 | 15 | 69.5 |
| 9 | CN | CN | EtBr | 50 | 8 | 95.4 |
| 10 | CN | CN | n-PrBr | 50 | 8 | 95.3 |
| 11 | CN | CN | n-BuBr | 50 | 8 | 95.6 |
| 12 | CN | CO2Et | PhCH2Br | 60 | 8 | 94.4 |
| 13 | CN | CO2Et | n-PrBr | 60 | 8 | 90.6 |
| 14 | CN | CO2Et | n-BuBr | 60 | 8 | 89.5 |
| 15 | MeCO | CO2Et | n-HexBr | 70 | 10 | 80.8 |
| 16 | MeCO | CO2Et | n-BuBr | 70 | 10 | 83.2 |
| 17 | MeCO | CO2Et | EtBr | 70 | 10 | 85.3 |
To further explore the scope and limitations of this method, various active methylene compounds (W1, W2 = CO2Et, CN, MCO, Ph) and alkyl halide were also examined (Table 3). Surprisingly, in all cases, the products of O-alkylation and dialkylation that occurred when using usual base were not detected.11
The reaction system exhibited higher chemical and regioselectivity in good and excellent yields. Thus, the less active compound phenylacetonitrile, as the active methylene compound, could also enable alkylation (Table 3, entries 7 and 8). It's worth noting that diethyl 2-(3-chloropropyl)malonate, a useful intermediate of synthetic melotonin, was selectively synthesized by the reaction of diethyl malonate with 1-bromo-3-chloropropane at 65 °C and gave 87.5% yield (Table 3, entry 5). By contrast, the yield was only 60.2% using NaOEt as base.
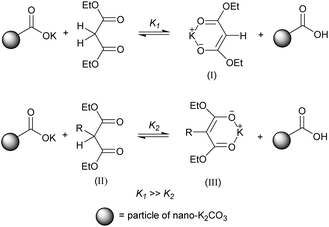
Reaction selectivity can be ascribed to three factors. First, although nano-K2CO3 had a stronger basic property than normal K2CO3 in ethanol, the former was still a weaker base than sodium ethoxide. Thus, the equilibrium constant K1 was far greater than the equilibrium constant K2. Second, the pKa of active methylene compound was greater than that of active methylene compound at α-carbon alkylation. Third, the electronic and steric effects of substitutents led to easier reaction with nano-K2CO3 particle to form enolic ion (I) than (II) (Scheme 1). Thus, the monoalkylated product could be selectively produced using nano-K2CO3 as base.
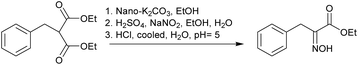
Furthermore, the oximation of monosubstituted and nonsubstituted active methylene compounds were examined. Diethyl benzylmalonate was selected as a model substrate for the optimization of oximation conditions (Table 4). Ethyl nitrite, which was slowly generated by the reaction of sodium nitrite with ethanol in the presence of sulfuric acid, was directly introduced into the ethanol suspension solution of nano-K2CO3 and diethyl benzylmalonate to give the corresponding oximation product. However, using normal K2CO3 as base, no reaction was detected. The reaction conditions were optimized by assessing the amount of base and sodium nitrite (sulfuric acid), reaction temperature and time for obtaining the maximum yield. The yield was improved with temperatures raised. But, boiling point of ethyl nitrite is just 17.4 °C, high temperature make ethyl nitrite exist in the form of gas. So the reaction temperature was controlled at 10 °C (Table 4, entries 1–4). Increasing reaction time had no significant for improvement of the yield (Table 4, entry 5). Increasing the amount of ethyl nitrite had significant improvement on the reaction (Table 4, entries 6, 7). Increasing the amount of nano-K2CO3 had significant improvement to the yield in 0.3–0.5 mol (Table 4, entries 9, 10). Obviously, this synthetic strategy (Table 4, entry 10) compared with traditional solution protocol10b has advantages of high yield, mild reaction condition and simplified work-up process.
| Entry | Nano-K2CO3 (mol) | NaNO2 (H2SO4)b (mol) | T (°C) | Time (h) | Yieldc (%) |
|---|---|---|---|---|---|
| a Diethyl benzylmalonate was 0.2 mol.b Ethyl nitrite was generated by the reaction of sodium nitrite with ethanol in the presence of sulfuric acid.c Yield of isolated product. | |||||
| 1 | 0.3 | 0.2 (0.1) | 0 | 5 | 69.3 |
| 2 | 0.3 | 0.2 (0.1) | 5 | 5 | 75.9 |
| 3 | 0.3 | 0.2 (0.1) | 10 | 5 | 80.5 |
| 4 | 0.3 | 0.2 (0.1) | 13 | 5 | 70.2 |
| 5 | 0.3 | 0.2 (0.1) | 10 | 6 | 80.8 |
| 6 | 0.3 | 0.25 (0.125) | 10 | 5 | 85.7 |
| 7 | 0.3 | 0.3 (0.15) | 10 | 5 | 89.7 |
| 8 | 0.3 | 0.4 (0.2) | 10 | 5 | 89.8 |
| 9 | 0.4 | 0.3 (0.15) | 10 | 5 | 91.6 |
| 10 | 0.5 | 0.3 (0.15) | 10 | 5 | 94.5 |
| 11 | 0.6 | 0.3 (0.15) | 10 | 5 | 94.5 |
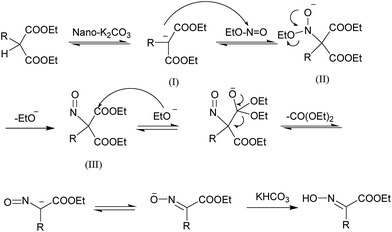
The possible mechanism is represented in Scheme 2. The enolic ion (I) generated by nano-K2CO3 reacts with ethyl nitrite to afford nitroso derivative (II). Sequentially, the intermediate (II) is cleaved by ethoxyl anion derived from the ethyl nitrite to form the oximino ester and diethyl carbonate.
Oximations of various typical β-dicarbonyl compounds were investigated (Table 5). Both monosubstituted and nonsubstituted active methylene compounds could be oximated in good or excellent yields. Arylmethyl (Table 5, entries 1–3) and aryl (Table 5, entry 4) substituted malonic ester could be oximated to afford corresponding products in excellent yields. For dicarbonyl compounds without substituent group at methylene, such as malonate ester and β-ketocarboxylate ester, oximation reactions also smoothly proceeded (Table 5, entries 5–7).
After alkylation reaction completion, the mixture of K2CO3 and potassium halide were not separated easily. The regeneration of nano-K2CO3 used in oximation reaction was carried out. After reaction completion, the filter cake was calcined at 250 °C over 4 h to generate normal K2CO3 with yield ≥95%. The normal K2CO3 was milled again to generate nano-K2CO3 with average particle size of 64 nm. The reaction activity of recovered nano-K2CO3 was evaluated through repeating oximation of diethyl benzylmalonate and the yield kept constant when the experiment was carried out repeatedly 10 times. Hence, the nano-K2CO3 exhibits the same activity within 10 times at least.
Conclusions
In summary, a relatively high activity, low cost, and reusable base nano-K2CO3 was developed. The material was endowed with a small average particle size, narrow particle size distribution and strong basicity. Nano-K2CO3 can replace sodium ethoxide to complete monoalkylation and oximation of various active methylene compounds in good or excellent yield. Further application of nano-K2CO3 will be reported shortly.Experimental section
General remarks
All commercially reagents were used without further purification. 1H NMR and 13C NMR spectra were recorded with a Bruker Advance II 500 instrument at 500 and 126 MHz, respectively. Chemical shifts were given as δ values (ppm), with tetramethylsilane as internal standard. Coupling constants (J) were given in hertz (Hz). The particle size of nano-K2CO3 was measured using a laser particle size analyzer (Zetasizer Nano S90, Malvern Instruments Ltd.). CO2-TPD was measured with Chemisorb 2720 automatic chemical adsorption apparatus (Micromeritics Instrument Corp). Nano-K2CO3 was prepared using GZM-5 High Frequency Resonant Grinding Machine (Beijing More Open Source Technology Development Ltd., Beijing, China) (47.8 Hz) and was observed by scanning electronic microscopy (SEM) performed on a LEO 1530VP instrument.Preparation of nano-K2CO3
Anhydrous K2CO3 (150 g), absolute ethanol (63 mL), and lauric acid (0.435 g) were poured into a resonance mill. The mixture was milled at room temperature for 8 h and then directly used for the next reaction.Typical procedure for the alkylation of active methylene compounds
The other monoalkylated products were prepared similarly according to the procedure used for diethyl 2-(3-chloropropyl) malonate.
Typical procedure for the oximation of β-dicarbonyl compounds
The other oximes were prepared similarly according to the procedure used for ethyl 2-(hydroxyimino)-3-phenylpropanoate.
Regeneration of nano-K2CO3
After oximation reaction completion, the mixture were filtered and washed with ethanol (3 × 30 mL). The filter cake was calcined in muffle at 250 °C for 4 hours to generate normal K2CO3 with ≥95% yield. The normal K2CO3 was milled again as the procedure of preparation of nano-K2CO3. Nano-K2CO3 was obtained and the average particle size of the particles was still 64 nm measured by laser particle size analyzer.Acknowledgements
We are grateful to the National Basic Research Program of China (2011CB512007, 2012CB723501) and the Hebei Natural Science Foundation (No. 12966737D).Notes and references
- (a) L. L. Chng, N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2013, 46, 1825 CrossRef CAS PubMed; (b) S. Shylesh, V. Schuenemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- (a) Z. Zarnegar and J. Safari, RSC Adv., 2014, 4, 20932 RSC; (b) Y. Wang, A. V. Biradar, C. T. Duncan and T. Asefa, J. Mater. Chem., 2010, 20, 7834 RSC; (c) X. Wang, D. Liu, S. Song and H. Zhang, J. Am. Chem. Soc., 2013, 135, 15864 CrossRef CAS PubMed.
- (a) S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 1754 CrossRef CAS; (b) A. Saxena, A. Kumar and S. Mozumdar, J. Mol. Catal. A: Chem., 2007, 269, 35 CrossRef CAS; (c) L. Hu, X.-Q. Cao, L.-Y. Shi, F.-Q. Qi, Z.-Q. Guo, J.-M. Lu and H.-W. Gu, Org. Lett., 2011, 13, 5640 CrossRef CAS PubMed; (d) V. K. Das and A. J. Thakur, Tetrahedron Lett., 2013, 54, 4164–4166 CrossRef CAS; (e) S.-S. Liu, K.-Q. Sun and B.-Q. Xu, ACS Catal., 2014, 4, 2226 CrossRef CAS; (f) R. B. N. Baig and R. S. Varma, Chem. Commun., 2012, 48, 2582 RSC; (g) E. Yoo, T. Okata, T. Akita, M. Kohyama, J. Nakamura and I. Honma, Nano Lett., 2009, 9, 2255 CrossRef CAS PubMed.
- (a) H. R. Momenian, S. Gholamrezaei, M. Salavati-Niasari, B. Pedram, F. Mozaffar and D. Ghanbari, J. Cluster Sci., 2013, 24, 1031 CrossRef CAS; (b) Y. He, Appl. Surf. Sci., 2006, 252, 2115 CrossRef CAS; (c) W. Sun, R. Gao and K. Jiao, J. Phys. Chem. B, 2007, 111, 4560 CrossRef CAS PubMed; (d) D. Shan, S. Wang, H. Xue and S. Cosnier, Electrochem. Commun., 2007, 9, 529 CrossRef CAS; (e) S. Mishra, S. H. Sonawane and R. P. Singh, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 107 CrossRef CAS.
- (a) B. Maleki, S. Barat Nam Chalaki, S. Sedigh Ashrafi, E. Rezaee Seresht, F. Moeinpour, A. Khojastehnezhad and R. Tayebee, Appl. Organomet. Chem., 2015, 29, 290 CrossRef CAS; (b) F. Moeinpour and A. Khojastehnezhad, Chin. Chem. Lett., 2015, 26, 575 CrossRef CAS; (c) Z. He, L. Xiong, J. Wang, J. Qiu and X. Fu, Mater. Sci. Forum, 2011, 694, 98 CrossRef CAS.
- (a) J. Li, J. Wang and S. Liu, Fine Chem., 2012, 29, 794 Search PubMed; (b) S. Fan, S. Liu, H. Zhang, Y. Liu, Y. Yang and L. Jin, Eur. J. Org. Chem., 2014, 5591 CrossRef CAS.
- Z. Bujnakova, P. Balaz, P. Makreski, G. Jovanovski, M. Caplovicova, L. Caplovic, O. Shpotyuk, A. Ingram, T.-C. Lee, J.-J. Cheng, J. Sedlak, E. Turianicova and A. Zorkovska, J. Mater. Sci., 2015, 50, 1973 CrossRef CAS.
- (a) M. Ohashi, K. Nakatani, H. Maeda and K. Mizuno, J. Photochem. Photobiol., A, 2010, 214, 161 CrossRef CAS; (b) S. Arumugam, D. McLeod and J. G. Verkade, J. Org. Chem., 1998, 63, 3677 CrossRef CAS.
- (a) C. Mordant, P. Duenkelmann, V. Ratovelomanana-Vidal and J.-P. Genet, Eur. J. Org. Chem., 2004, 3017 CrossRef CAS; (b) S. Liu, Y. Yang, X. Zhen, J. Li, H. He, J. Feng and A. Whiting, Org. Biomol. Chem., 2012, 10, 663 RSC; (c) Y. Yang, S. Liu, J. Li, X. Tian, X. Zhen and J. Han, Synth. Commun., 2012, 42, 2540 CrossRef CAS.
- (a) J. H. M. Lange, M. A. W. van der Neut, H. C. Wals, G. D. Kuil, A. J. M. Borst, A. Mulder, A. P. den Hartog, H. Zilaout, W. Goutier, H. H. van Stuivenberg and B. J. van Vliet, Bioorg. Med. Chem. Lett., 2010, 20, 1084 CrossRef CAS PubMed; (b) S. Liu, Y. Mu, J. Han, X. Zhen, Y. Yang, X. Tian and A. Whiting, Org. Biomol. Chem., 2011, 9, 7476 RSC; (c) J. P. Street, Tetrahedron Lett., 1991, 32, 3333 CrossRef CAS.
- (a) S. J. Rhoads and R. W. Holder, Tetrahedron, 1969, 25, 5443 CrossRef CAS; (b) A. V. Bedekar, T. Watanabe, K. Tanaka and K. Fuji, Synthesis, 1995, 1069 CrossRef CAS; (c) T. Shono, S. Kashimura, M. Sawamura and T. Soejima, J. Org. Chem., 1988, 53, 907 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21597h |
| This journal is © The Royal Society of Chemistry 2016 |