Tough and strong nacre-like composites from hyperbranched poly(amido amine) and clay nanosheets cross-linked by genipin†
Wentao Haoa,
Liang Zhanga,
Xiaomin Wanga,
Jin Wanga,
Zhenhu Hub and
Wen Yang*a
aDepartment of Polymer Materials and Engineering, School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China. E-mail: wenyang@hfut.edu.cn
bDepartment of Municipal Engineering, School of Civil Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China
First published on 17th December 2015
Abstract
Tough and strong nacre-like composite films were fabricated from hyperbranched poly(amido amine) (HPAMAM) and clay nanosheets with the aid of genipin crosslinking. SEM observation and SAXS analysis indicated the genipin crosslinked HPAMAM/nanoclay composite films had a compact structure. However, DMA results showed that HPAMAM molecules still had excellent flexibility. FTIR analysis suggested that the HPAMAM molecules maintained their strong ability to form hydrogen bonds after crosslinking. Therefore, characteristics of sacrificial bonds were found. In addition, dual networks were thought to have been established inside the composite films, that is, a primary chemical network and a secondary physical network. Consequently, the genipin crosslinked HPAMAM/nanoclay composite films were shown to be tough and strong. The fracture toughness was as high as 5.03 MJ m−3 and the mechanical strength was about 152.9 MPa. Lastly, the good biocompatibility of all the components, including HPAMAM, nanoclay and genipin, give great opportunities for these composites to be widely applied in biomedical research.
1. Introduction
The demand for strong and tough composites has driven researchers to develop various strategies. Among them, biomimicking is of particular interest.1–6 Nowadays, a great many nacre-like composites with very high mechanical strengths have been reported.7–12 However, materials with good toughness (fracture toughness ≥ 2 MJ m−3) are still highly demanded.10,13–17 Detailed analysis of natural composites, including nacre and bone, indicates that sacrificial bonds are essential to the toughness of these materials.18–22 Sacrificial bonds are weak, reversible interactions, for example, hydrogen bonds, ionic bonds and π–π interactions.23–25Recent reports showed that it was effective to introduce sacrificial bonds into artificial bulk composites to improve their toughness.26–28 Lots of energy can be dissipated as the hydrogen bonds26 or π–π interactions27,28 rupture. Nevertheless, such an idea has not been widely adopted in fabrication of high toughness nacre-like composites.29–31 One possible reason might be the lack of proper materials to function as sacrificial bonds donor and acceptor. Although the linear poly(vinyl alcohol) can form hydrogen bonds among the molecular chains, their mobility is greatly restricted when confined between the densely packed clay nanosheets.32,33
Fortunately, the dentric aliphatic polymers, including hyperbranched polyglycerol34,35 and poly(amido amine),36 were shown to be of great potential in establishing sacrificial bonds in the nacre-like composites and thus improving the toughness of these composites. The hyperbranched aliphatic polymers are very easily to be synthesized from common monomers. They are always able to form inter- and intra-molecular hydrogen bonds.37–40 Besides, the hyperbranched aliphatic polymers are of strong molecular mobility, which is very favourable for the hydrogen bonds to exchange quickly and reversibly,38 because of their small molecular size, weak entanglement, and lots of inner cavities. In our previous report,36 we constructed a kind of ductile nacre-like composites from HPAMAM and clay nanosheets (elongation at break ≥30%). However, their tensile strength was less than 20 MPa. Perhaps it is because there are no strong entanglements among the hyperbranched polymers.
Crosslinking is an effective way in improving the strength of composite materials through enhancing the interactions among components.8,35 By carefully selecting the crosslinkers and finely adjusting their amounts, one might obtain nacre-like composites that are both tough and strong from hyperbranched polymers and clay nanosheets. Genipin, a derivative of natural product, is of good biocompatibility.41 It has been applied in many biomaterials to crosslink polymers with amine groups, for example, collagen42 and chitosan.43,44 Because there are abundant amine groups on the HPAMAM molecules, the genipin can react with HPAMAM well. Furthermore, the obtained composites may inherent the good biocompatibility of genipin and the HPAMAMs, which have already been applied in biomedical applications.45–49
Here in this report, we demonstrated that tough and strong nacre-like composites were obtained from HPAMAM and clay nanosheets after crosslinking. The HPAMAM/nanoclay composites were prepared first and subsequently immersed in genipin aqueous solutions to fulfil the crosslinking reactions. The maximum fracture toughness of the nacre-like composites was as high as 5.03 MJ m−3, far beyond that of the natural nacre. Compared with the previous report,36 mechanical strength of the nacre-like composites was improved from ∼20 MPa to 152.9 ± 7.6 MPa, nearly 8 times increment. The HPAMAM/nanoclay composite films were turned from sole ductile to tough and strong. A dual-network was thought having been be established inside the composite films, which was the key to the excellent mechanical properties of the HPAMAM/nanoclay composite films.
2. Experimental sections
2.1. Materials
N,N′-Methylene diacrylamide (MBA, 99.5%) and N-aminoethylpiperazine (AEPZ, 99.5%) were products from Aladdin Reagents Co., Ltd. Nanoclay (diameter 20–30 nm, thickness ∼1 nm) was purchased from Hangzhou Xihe Chemicals Co., Ltd. Genipin (98%) was a product of Chendu Conbon Bio-tech Co., Ltd. Methanol and acetone were purchased from Sino-pharm Co., Ltd.2.2. Preparation of genipin crosslinked HPAMAM/nanoclay nacre-like composites
HPAMAM/nanoclay nacre-like composites were prepared in the same procedure reported previously.36,50 Briefly, the HPAMAM was first synthesized from MBA and AEPZ in a molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a mixture solvent of methanol and water (v
1 in a mixture solvent of methanol and water (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 7
v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The molecular weight of HPAMAM was about 8.2 × 103 g mol−1. Before use, the obtained HPAMAM was precipitated twice in cold acetone. Clay nanosheets were dispersed in deionized water and violently stirred for a week to fully exfoliate them. Equal volume of aqueous solutions of HPAMAM (2 wt%) and nanoclay dispersions (0.5 wt%) were mixed together and gently stirred for a period of time. After being centrifuged at 9000 rpm, the hybrids of HPAMAM and nanoclay were collected and re-dispersed in deionized water. Later, the dispersions were filtrated through the cellulose filtrate paper (pore size is 0.22 μm) with the aid of vacuum. Finally, the products were evaporated under room temperature for 2–3 days. Weight fraction of nanoclay was determined by TGA, which was about 55–57%.
3). The molecular weight of HPAMAM was about 8.2 × 103 g mol−1. Before use, the obtained HPAMAM was precipitated twice in cold acetone. Clay nanosheets were dispersed in deionized water and violently stirred for a week to fully exfoliate them. Equal volume of aqueous solutions of HPAMAM (2 wt%) and nanoclay dispersions (0.5 wt%) were mixed together and gently stirred for a period of time. After being centrifuged at 9000 rpm, the hybrids of HPAMAM and nanoclay were collected and re-dispersed in deionized water. Later, the dispersions were filtrated through the cellulose filtrate paper (pore size is 0.22 μm) with the aid of vacuum. Finally, the products were evaporated under room temperature for 2–3 days. Weight fraction of nanoclay was determined by TGA, which was about 55–57%.
The obtained HPAMAM/nanoclay nacre-like composites were immersed in the 20 mL aqueous solution of genipin at room temperature. The concentrations were set to be 0.025%, 0.05%, 0.1% and 0.2%. Treatment time was 6 hours. After that, the composite films were taken out, washed with deionized water for 3 times. Lastly, these films were dried at room temperature for 2–3 days. During immersion, the nacre-like composites turned from colourless to dark blue, indicating chemical reactions occurred between HPAMAM and genipin.41–43
2.3. Characterization
FTIR spectra of the nacre-like composites were recorded on Nicolet iS10 (Thermo Scientific) in ATR mode. UV-vis spectra were recorded on an UV-2550 (Shimazu). SAXD was performed on a Rigaku D/max-TTRIII (CuK target, λ = 0.154184 nm). SEM observation was performed on a Sirion200 (FEI). Samples were adhered onto the side surface of supporting poles. The cross-sectional fracture surfaces of tensile test samples were observed. TGA measurement was done on a Netsch TG209 from room temperature to 800 °C in air. The heating rate was 10 °C min−1. Mechanical properties were measured on a universal testing machine manufactured by Shenzhen SANS testing machine Co., Ltd. Dynamic mechanical analysis (TA Instruments Q800) was performed in the temperature ramp mode at a heating rate of 5 °C min–1.3. Results and discussion
3.1. Morphology of genipin crosslinked HPAMAM/nanoclay hybrid films
Although the pristine HPAMAM/nanoclay nacre-like composites were colourless and highly transparent as shown in Fig. 1a, the genipin crosslinked nacre-like composites (G-HPAMAM/nanoclay) were no longer colourless. They were dark blue in appearance (Fig. 1b). The darker colour indicated that some reactions occurred between HPAMAM and genipin.41–43 | ||
| Fig. 1 Optical pictures (a, b) and FTIR spectra of pristine and genipin crosslinked HPAMAM/nanoclay composite films. (c) Genipin aqueous concentration was 0.025%. | ||
UV-vis spectra of the obtained film showed that there was an absorption peak centred at 610 nm, while there was no such absorption for the non-crosslinked HPAMAM/nanoclay composite film or the genipin aqueous solution (Fig. S1†).
As shown on the FTIR spectrum of genipin crosslinked HPAMAM/nanoclay composite film (Fig. 1c), the peak at 1278 cm−1 was attributed to the C–O–C stretch of ester groups on the genipin molecules.51 It suggested the presence of genpin in the nacre-like composites. Moreover, the vibration peak at around 1540 cm−1 was weakened, and the peak at 1645 cm−1 became relative stronger. Such results suggested the formation of an amide linkage between genipin and HPAMAM.41,52
3.2. Microstructure of genipin crosslinked HPAMAM/nanoclay nacre-like composites
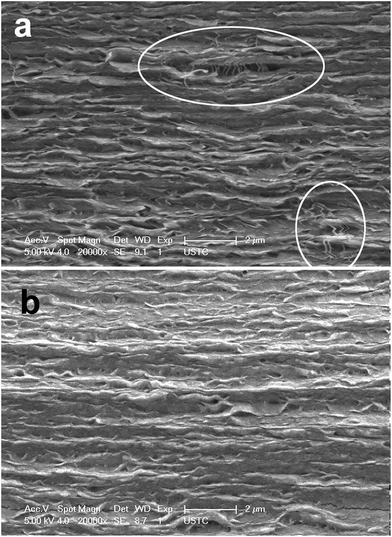
From the SEM images in Fig. 2a and b (observation on the cross-section of HPAMAM/nanoclay composites after tensile test), it could be seen that the HPAMAM/nanoclay nacre-like composites showed highly ordered structure, similar to the natural nacre and those nacre-like composites.53,54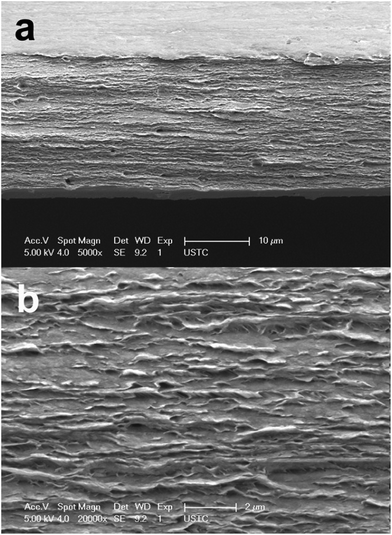 | ||
Fig. 2 SEM images of fracture surfaces of genipin crosslinked HPAMAM/nanoclay composite film: (a) 5000×; (b) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. Concentration of genipin aqueous solution was 0.025%. 000×. Concentration of genipin aqueous solution was 0.025%. | ||
More interestingly, there were some other unique features. It could be seen in Fig. 2a that the cross-section area was reduced. The top and bottom edge curved to the centre of the fracture surface. In addition, apparent “pulling out”14 (the white sheets outside the fracture surface) were found in Fig. 2b. These results indicated that plastic flow had occurred during tensile test.
Generally, the area reduction of cross-section is the result of shear-yielding, characteristics of ductile fracture. The “pulling out” phenomenon was evidence of sliding, which could effectively detain the fracture of the nacre-like composites.14 A lot of energy could be dissipated before the nacre-like composites failed. Results from SEM observation strongly suggested that the G-HPAMAM/nanoclay nacre-like composites were of high toughness. There might be two reasons for the unique morphology (area reduction and pulling out) of the HPAMAM/nanoclay nacre-like composites: (1) the HPAMAM moelcules were very flexible; (2) there was strong interlock among the HPAMAM molecules and the clay nanosheets. To find more clues to the toughness of the G-HPAMAM/nanoclay nacre-like composites, dynamic mechanical analysis and small angle diffraction were done.
As it could be seen in Fig. 3a, there were two relaxation peaks on the loss modulus curves of G-HPAMAM/nanoclay nacre-like composites. One was located at about −40 °C, and another one was at around 7 °C. The relaxation peak at −40 °C indicated the strong mobility of secondary motion units on the HPAMAM molecules. The relaxation peak at 7 °C was broad, suggesting a wide distribution of relaxation times of the polymer segments.32 Although the peak at 7 °C of the composite film was suppressed as higher amount of genipin was used, the relative strength of peak at −40 °C was still strong. The HPAMAM molecules kept their excellent flexibility and mobility even being confined in the small rooms between the clay nanosheets and after crosslinking. Such properties might be attributed to their unique molecular structures, that is, the highly branched structure with many inner cavities.
In the previous report, the “pulling out” was thought to be the result of strong marginal interlock that the graphene nanosheets had to undergo large deformation before failure.14 Here in this work, this phenomenon was attributed to be the strong interaction between HPAMAM and clay nanosheets. The flexible HPAMAM molecules were pulled out accompanying the clay platelets. Some clues could be found from the SAXD patterns, as shown in Fig. 3b. The diffraction peak of G-HPAMAM/nanoclay shifted to higher degree than that of the non-crosslinked composite, indicating that the interlayer distances of clay nanosheets were reduced. After crosslinking, the HPAMAM molecules were tightly bound together, resulting in shortened spacing of the clay nanosheets. The mutual interactions between HPAMAM and nanoclay were subsequently enhanced.
From the morphology observation, one can image that chemical networks have been built in the genipin crosslinked HPAMAM/nanoclay nacre-like composites. The HPAMAM molecules and the clay nanosheets are all locked together by genipin molecules. The results might be: flow of HPAMAM molecules was suppressed; stress can be more effectively transferred between the clay nanosheets. It hints that the toughness and strength of the HPAMAM/nanoclay nacre-like composites can both be improved.
3.3. Mechanical performance of genipin crosslinked HPAMAM/nanoclay nacre-like composites
As shown in Fig. 4a and b, the genipin crosslinked HPAMAM/nanoclay nacre-like composites were very flexible. They could be horizontally folded without being damaged, showing the excellent flexibility of the HPAMAM molecules. Besides, the nacre-like composites were strong. A tiny film that was only 50 μm thick and 7 mm wide could easily lifted up 400 g (2 × 200 gram) weight (the stress applied was 11.2 MPa). | ||
| Fig. 4 Illustration to flexibility and robustness of G-HPAMAM/nanoclay nacre-like composites. The composites had been treated with a 0.025% genipin solution. | ||
The results of tensile test were collected in Table 1. The fracture toughness was calculated by integrating the area under the stress–strain curves. Genipin crosslinking lead to improved fracture toughness. When the composites were treated with 0.025% genipin solution, the fracture toughness was 5.03 MJ m−3, 1.27 times that of the pristine one. Moreover, it was nearly 2.8 times that of the natural nacre (1.8 MJ m−3).14 The tensile strength was improved too. It was 55.3 MPa for the same composite, nearly 200% that of the pristine one. Surprisingly, the Young's modulus was almost 10 times higher. Crosslinking showed enormous effect on the mechanical properties of HPAMAM/nanoclay composite films. As illustrated in Fig. 3, crosslinking by genipin greatly suppressed the plastic flow of HPAMAM. Moreover, crosslinking remarkably enhanced the mutual interactions between the polymer and clay nanoplatelets, and restricted the slide between the clay nanosheets.8 Therefore, the strength and moduli of the HPAMAM/nanoclay composites were dramatically improved.
| Concentration of genipin aqueous solution (%) | Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) | Fracture toughness (MJ m−3) |
|---|---|---|---|---|
| 0 | 18.6 ± 3.0 | 0.37 ± 0.05 | 30.9 ± 0.6 | 3.95 ± 0.45 |
| 0.025 | 55.3 ± 3.1 | 3.43 ± 0.23 | 12.2 ± 2.2 | 5.03 ± 0.52 |
| 0.05 | 85.3 ± 1.4 | 4.63 ± 0.22 | 7.85 ± 4.11 | 4.21 ± 1.84 |
| 0.1 | 106.2 ± 5.5 | 5.02 ± 0.19 | 3.48 ± 0.54 | 2.01 ± 0.40 |
| 0.2 | 152.9 ± 7.6 | 6.46 ± 0.38 | 3.48 ± 0.08 | 2.31 ± 0.28 |
As the HPAMAM/nanoclay nacre-like composites were treated with higher amount of genipin, fracture toughness of the composites decreased. However, even the lowest one was larger than that of the natural nacre. These nacre-like composites were still very tough. Meanwhile, the tensile strength of G-HPAMAM/nanoclay composites increased drastically with more genipin was applied. The modulus increased gradually too. That is, the G-HPAMAM/nanoclay composites were becoming much stronger. The ultimate tensile strength of the composites could reach as high as 152.9 MPa, over 8 times that of the non-crosslinked one. The tensile strength was not only comparable to that of the natural nacre (78–130 MPa, wet; 90–170 MPa, dry),55,56 but also comparable to that of the marginally crosslinked graphene papers (156.8 MPa)14 or borate crosslinked graphene oxide films (160 ± 18 MPa).57 Tough and strong nacre-like composites were obtained through genipin crosslinking the relative weak HPAMAM/nanoclay hybrid films.
3.4. Influences of genipin crosslinking on the HPAMAM/nanoclay nacre-like composites
Typical tensile stress–strain curves of genipin crosslinked HPAMAM/nanoclay nacre-like composites were shown in Fig. 5. With the genipin concentration increasing, the G-HPAMAM/nanoclay composite turned from soft and ductile to hard and strong. For those slightly crosslinked composite films, the most important feature was that sequential transitions appeared on the stress–strain curves (see Fig. 5, line 2 and line 3; see also Fig. S3a†). It suggested that rupture of sacrificial bonds had occurred sequentially.26 However, the sequential transitions disappeared on the stress–strain curves of densely crosslinked composite samples (Fig. 5, line 5; Fig. S3b†). | ||
| Fig. 5 Typical tensile stress–strain curves of HPAMAM/nanoclay nacre-like composites. As the arrows indicated, sequential transitions can be found on slightly crosslinked samples. | ||
As we took a close look to the fracture morphology of the slightly crosslinked G-HPAMAM/nanoclay composites shown in Fig. 6a, interesting things were found. There were some fibrils bridging the cracks. The fibrils were similar to the hidden lengths in the natural composites, which were released after the sacrificial bonds broke down.22,58,59 Because the fibrils were several hundred nanometers long, much larger than that of single HPAMAM molecules, they must be bundles composed of many macromolecules. That is, the genipin crosslinked HPAMAMs. However, as relative higher concentration (0.2%) of genipin solutions were used, there was no fibril on the fracture surfaces (Fig. 6b). Instead, a much denser morphology was found.
FTIR of the G-HPAMAM/nanoclay composite films showed that the relative strength of hydrogen bonded amine groups kept almost constant (Fig. S4†). That is, the hydrogen bonds in the composite films were still abundant after crosslinking. These hydrogen bonds established a secondary network. For those slightly crosslinked samples, when the primary network (genipin crosslinked HPAMAMs) was extended under stress, the hydrogen bonding network ruptured first. Sequential transitions were found. During this process, a lot of energy was dissipated. However, the primary network became very tight when the composites were densely crosslinked. The primary network deformed simultaneously with the secondary network, resulting in extremely improved mechanical strength, whereas, the sequential transitions on the stress–strain curves disappeared.
4. Conclusions
In summary, a series of genipin crosslinked HPAMAM/nanoclay nacre-like composites were fabricated. These composites were tough and strong. The fracture toughness was high as 5.03 MJ m−3, and the tensile strength could reach 152.9 MPa. Considering that the mechanical strength of HPAMAM was only 5 MPa,36 and the non-crosslinked HPAMAM/nanoclay composites was about 18.6 MPa, the improvement was really impressive.HPAMAM molecules are of branched structure, lots of inner cavity and poor entanglement compared with the linear polymers. This kind of structure leads to strong mobility of the HPAMAM molecules, very favourable for the HPAMAMs to fabricate high toughness composites. Thanks to the numerous amine and amide groups, hydrogen bonding networks are naturally established among the HPAMAM molecules. The hydrogen bonds function well as sacrificial bonds which can detain the fatal rupture of materials, and much beneficial to the toughness. After crosslinking, a chemical network was imposed on the physical hydrogen bonding network, which greatly enhanced the interactions among HPAMAM molecules, in addition to that between HPAMAM and clay nanosheets. As a result, the genipin crosslinked HPAMAM/nanoclay composite films showed to be tough and strong.
Hyperbranched aliphatic polymers, including HPAMAM, hyperbranched poly(ester amide) and hyperbranched glycerol are all of strong flexibility and the ability to form hydrogen bonds. In addition, these polymers are of good biocompatibility. Therefore, the hyperbranched aliphatic polymers are very promising in construction of highly tough and strong composites to be applied in biomedical research, such as tissue engineering.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 21204016) and the Anhui Provincial Natural Science Foundation (No. 1508085ME107).Notes and references
- G. Mayer, Science, 2005, 310, 1144–1147 CrossRef CAS PubMed.
- H. B. Yao, J. Ge, L. B. Mao, Y. X. Yan and S. H. Yu, Adv. Mater., 2014, 26, 163–188 CrossRef CAS PubMed.
- Q. F. Cheng, L. Jiang and Z. Y. Tang, Acc. Chem. Res., 2014, 47, 1256–1266 CrossRef CAS PubMed.
- J. F. Wang, Q. F. Cheng and Z. Tang, Chem. Soc. Rev., 2012, 41, 1111–1129 RSC.
- A. R. Studart, Adv. Mater., 2012, 24, 5024–5044 CrossRef CAS PubMed.
- M. Darder, P. Aranda and E. Ruiz-Hitzky, Adv. Mater., 2007, 19, 1309–1319 CrossRef CAS.
- S. Deville, E. Saiz, R. K. Nalla and A. P. Tomsia, Science, 2006, 311, 515–518 CrossRef CAS PubMed.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. S. Shim, J. D. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80–83 CrossRef CAS PubMed.
- E. Munch, M. E. Launey, D. H. Alsem, E. Saiz, A. P. Tomsia and R. O. Ritchie, Science, 2008, 322, 1516–1520 CrossRef CAS PubMed.
- L. J. Bonderer, A. R. Studart and L. J. Gauckler, Science, 2008, 319, 1069–1073 CrossRef CAS PubMed.
- Z. Y. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS PubMed.
- A. Finnemore, P. Cunha, T. Shean, S. Vignolini, S. Guldin, M. Oyen and U. Steiner, Nat. Commun., 2012, 3, 966, DOI:10.1038/ncomms1970.
- P. Podsiadlo, Z. Q. Liu, D. Paterson, P. B. Messersmith and N. A. Kotov, Adv. Mater., 2007, 19, 949–955 CrossRef CAS.
- Q. F. Cheng, M. X. Wu, M. Z. Li, L. Jiang and Z. Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 3750–3755 CrossRef CAS PubMed.
- P. Podsiadlo, M. Michel, K. Critchley, S. Srivastava, M. Qin, J. W. Lee, E. Verploegen, A. J. Hart, Y. Qi and N. A. Kotov, Angew. Chem., Int. Ed., 2009, 48, 7073–7077 CrossRef CAS PubMed.
- J. F. Wang, Q. F. Cheng, L. Lin and L. Jiang, ACS Nano, 2014, 8, 2739–2745 CrossRef CAS PubMed.
- C. N. Wu, T. Saito, S. Fujisawa, H. Fukuzumi and A. Isogai, Biomacromolecules, 2012, 13, 1927–1932 CrossRef CAS PubMed.
- M. E. Launey, M. J. Buehler and R. O. Ritchie, Annu. Rev. Mater. Res., 2010, 40, 25–53 CrossRef CAS.
- H. B. Yao, H. Y. Fang, X. H. Wang and S. H. Yu, Chem. Soc. Rev., 2011, 40, 3764–3785 RSC.
- M. A. Meyers, J. McKittrick and P. Y. Chen, Science, 2013, 339, 773–779 CrossRef CAS PubMed.
- J. B. Thompson, J. H. Kindt, B. Drake, H. G. Hansma, D. E. Morse and P. K. Hansma, Nature, 2001, 414, 773–776 CrossRef CAS PubMed.
- G. E. Fantner, T. Hassenkam, J. H. Kindt, J. C. Weaver, H. Birkedal, L. Pechenik, J. A. Cutroni, G. A. G. Cidade, G. D. Stucky, D. E. Morse and P. K. Hansma, Nat. Mater., 2005, 4, 612–616 CrossRef CAS PubMed.
- G. E. Fantner, E. Oroudjev, G. Schitter, L. S. Golde, P. Thurner, M. M. Finch, P. Turner, T. Gutsmann, D. E. Morse, H. Hansma and P. K. Hansma, Biophys. J., 2006, 90, 1411–1418 CrossRef CAS PubMed.
- S. S. Nabavi, P. Fratzl and M. A. Hartmann, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2015, 91, 032603 CrossRef PubMed.
- C. K. C. Lieou, A. E. Elbanna and J. M. Carlson, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 88, 012703 CrossRef PubMed.
- J. R. McKee, J. Huokuna, L. Martikainen, M. Karesoja, A. Nykänen, E. Kontturi, H. Tenhu, J. Ruokolainen and O. Ikkala, Angew. Chem., Int. Ed., 2014, 53, 5049–5053 CrossRef CAS PubMed.
- M. J. Palmeri, K. W. Putz and L. C. Brinson, ACS Nano, 2010, 4, 4256–4264 CrossRef CAS PubMed.
- Z. X. Chen and H. B. Lu, J. Mater. Chem., 2012, 22, 12479–12490 RSC.
- P. Podsiadlo, A. K. Kaushik, B. S. Shim, A. Agarwal, Z. Y. Tang, A. M. Waas, E. M. Arruda and N. A. Kotov, J. Phys. Chem. B, 2008, 112, 14359–14363 CrossRef CAS PubMed.
- L. Martikainen, A. Walther, J. Seitsonen, L. Berglund and O. Ikkala, Biomacromolecules, 2013, 14, 2531–2535 CrossRef CAS PubMed.
- P. Das and A. Walther, Nanoscale, 2013, 5, 9348–9356 RSC.
- T. Verho, M. Karesoja, P. Das, L. Martikainen, R. Lund, A. Alegría, A. Walther and O. Ikkala, Adv. Mater., 2013, 25, 5055–5059 CrossRef CAS PubMed.
- J. F. Wang, Q. F. Cheng, L. Lin, L. F. Chen and L. Jiang, Nanoscale, 2013, 5, 6356–6362 RSC.
- X. Z. Hu, Z. Xu and C. Gao, Sci. Rep., 2012, 2, 767, DOI:10.1038/srep00767.
- X. Z. Hu, Z. Xu, Z. Liu and C. Gao, Sci. Rep., 2013, 3, 2374, DOI:10.1038/srep02374.
- W. Hao, S. Ding, L. Zhang, W. Liu and W. Yang, ChemPlusChem, 2014, 79, 211–216 CrossRef CAS.
- M. Gao, G. C. Kuang, X. R. Jia, W. S. Li, Y. Li and Y. Wei, Tetrahedron Lett., 2008, 49, 6182–6187 CrossRef CAS.
- Y. Lin, K. Y. Zhang, Z. M. Dong, L. S. Dong and Y. S. Li, Macromolecules, 2007, 40, 6257–6267 CrossRef CAS.
- I. Tanis and K. Karatasos, Phys. Chem. Chem. Phys., 2009, 11, 10017–10028 RSC.
- Y. Z. You, J. J. Yan, Z. Q. Yu, M. M. Cui, C. Y. Hong and B. J. Qu, J. Mater. Chem., 2009, 19, 7656–7660 RSC.
- F. Song and L. M. Zhang, Ind. Eng. Chem. Res., 2009, 48, 7077–7083 CrossRef CAS.
- Y. J. Hwang, J. Larsen, T. B. Krasieva and J. G. Lyubovitsky, ACS Appl. Mater. Interfaces, 2011, 3, 2579–2584 CAS.
- S. S. Silva, A. Motta, M. T. Rodrigues, A. F. M. Pinheiro, M. E. Gomes, J. F. Mano, R. L. Reis and C. Migliaresi, Biomacromolecules, 2008, 9, 2764–2774 CrossRef CAS PubMed.
- G. Wang, L. Zheng, H. Zhao, J. Miao, C. Sun, H. Liu, Z. Huang, X. Yu, J. Wang and X. Tao, ACS Appl. Mater. Interfaces, 2011, 3, 1692–1701 CAS.
- M. Sun, C. Y. Hong and C. Y. Pan, J. Am. Chem. Soc., 2012, 134, 20581–20584 CrossRef CAS PubMed.
- Y. Chen, L. Z. Zhou, Y. Pang, W. Huang, F. Qiu, X. L. Jiang, X. Y. Zhu, D. Y. Yan and Q. Chen, Bioconjugate Chem., 2011, 22, 1162–1170 CrossRef PubMed.
- W. Yang, C. Y. Pan, M. D. Luo and H. B. Zhang, Biomacromolecules, 2010, 11, 1840–1846 CrossRef CAS PubMed.
- Y. J. Tsai, C. C. Hu, C. C. Chu and T. Imae, Biomacromolecules, 2011, 12, 4283–4290 CrossRef CAS PubMed.
- W. Yang, C. Y. Pan, X. Q. Liu and J. Wang, Biomacromolecules, 2011, 12, 1523–1531 CrossRef CAS PubMed.
- W. Hao, X. Wang, S. Ding, Y. Cao, H. Zhang and W. Yang, RSC Adv., 2015, 5, 86861–86866 RSC.
- H. G. Sundararaghavan, G. A. Monteiro, N. A. Lapin, Y. J. Chabal, J. R. Miksan and D. I. Shreiber, J. Biomed. Mater. Res., Part A, 2008, 87, 308–320 CrossRef PubMed.
- A. Khan, S. Salmieri, C. Fraschini, J. Bouchard, B. Riedl and M. Lacroix, ACS Appl. Mater. Interfaces, 2014, 6, 15232–15242 CAS.
- H. B. Yao, H. Y. Fang, Z. H. Tan, L. H. Wu and S. H. Yu, Angew. Chem., Int. Ed., 2010, 49, 2140–2145 CrossRef CAS PubMed.
- A. Walther, I. Bjurhager, J.-M. Malho, J. Ruokolainen, L. Berglund and O. Ikkala, Angew. Chem., Int. Ed., 2010, 49, 6448–6453 CrossRef CAS PubMed.
- J. Sun and B. Bhushan, RSC Adv., 2012, 2, 7617–7632 RSC.
- H. Kakisawa and T. Sumitomo, Sci. Technol. Adv. Mater., 2011, 12, 064710, DOI:10.1088/1468-6996/12/6/064710.
- Z. An, O. C. Compton, K. W. Putz, L. C. Brinson and S. T. Nguyen, Adv. Mater., 2011, 23, 3842–3846 CAS.
- X. Q. Li and H. C. Zeng, Adv. Mater., 2012, 24, 6277–6282 CrossRef CAS PubMed.
- Z. H. Xu and X. D. Li, Adv. Funct. Mater., 2011, 21, 3883–3888 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21580c |
| This journal is © The Royal Society of Chemistry 2016 |