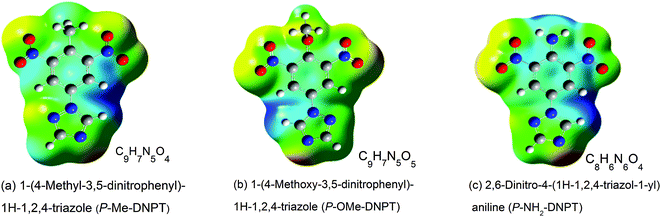
Evaluation of thermal stability and acoustic fingerprint spectra of energetic 1,2,4-triazoles based on bond lengths of chemical substituents using pulsed photoacoustic technique
K. S. Raoa,
A. K. Chaudhary*a,
N. Kommua and
A. K. Sahooab
aAdvanced Centre of Research in High Energy Materials, University of Hyderabad, Hyderabad-500 046, India. E-mail: anilphys@yahoo.com; akcphys@gmail.com
bSchool of Chemistry, University of Hyderabad, Hyderabad-500 046, India
First published on 21st December 2015
Abstract
We investigated the effect of the bond lengths of the chemical substituents (attached to the para position of the phenyl ring) on the thermal stability of three newly synthesized nitro rich 1,2,4-triazoles, namely, 1-(4-methyl-3,5-dinitrophenyl)-1H-1,2,4-triazole (P-Me-DNPT), 1-(4-methoxy-3,5-dinitrophenyl)-1H-1,2,4-triazole (P-OMe-DNPT) and 2,6-dinitro-4-(1H-1,2,4-triazol-1-yl)aniline (P-NH2-DNPT). The thermal stability of these compounds was evaluated along with their acoustic fingerprint spectra between 30 and 350 °C, using a pulsed photoacoustic (PA) pyrolysis technique. A 532 nm wavelength of pulse duration 7 ns and repetition rate of 10 Hz, obtained from a Q-switched Nd:YAG laser, was used to determine the released NO2 molecules during the process of thermal decomposition. The thermo gravimetric-differential thermal analysis (TG-DTA) data along with PA results highlight the multistep decomposition mechanism of 1,2,4-triazoles. The study also helps us to distinguish the characteristic behavior of the reported molecules as propellants and explosives for rocket fuels.
1. Introduction
Nitro-rich tetrazoles, triazoles, pyrazoles and their derivatives are newly synthesized high energy materials (HEMs), which possess high heats of formation, detonation velocities, pressure, shock sensitivity, and good thermal stability due to the presence of the aromatic ring.1–3 The synthesis and characterisation of these HEMs molecules have attracted many researchers to evaluate their potential in military applications such as rocket fuels, gun propellants and explosives.4–10 In addition, the study of the thermal stability and decomposition mechanisms of premier HEMs molecules have been reported by several research groups using different analytical techniques.11–15 The photoacoustic (PA) technique is very versatile and offers several advantages such as high selectivity, sensitivity, fast response time, and the equipment is compact in size. As a result, it is widely used in trace gas detection, from ppb to ppt range.16–20 Furthermore, the significance of the PA signal is realised in monitoring the released gaseous molecules from solid explosives available in small quantities (∼1 mg) during the pyrolysis process. This is achieved by means of selecting appropriate excitation wavelengths and designing the PA cell along with the heating system. Therefore, the PA pyrolysis technique is one of the emerging analytical techniques for the study of the thermal decomposition and stability of newly synthesized HEMs molecules. In the present case, we investigated the role of –NO2, a major functional group in the thermal decomposition mechanism of compounds in terms of the strength of the PA signal and the excited acoustic modes of the PA cavity. The released mechanism of gaseous molecules is ascertained on the basis of the bond length of the chemical substituents present in the compounds.Kommu et al. reported the synthesis of nitro rich 1,2,4-triazole derivatives such as P-Me-DNPT, P-OMe-DNPT and P-NH2-DNPT.21 The structures along with the chemical formulas of these compounds are shown in Fig. 1. The phenyl ring of these compounds contain two NO2 groups at their meta positions and the para positions are occupied by methyl, methoxy and amino groups. The estimated values of the densities (ρ) and detonation velocities (D) of these compounds are 1.62, 1.64, 1.66 g cm−3 and 6.40, 6.68, 6.66 km s−1, respectively. The detonation velocity of P-OMe-DNPT has slightly higher value than the others. This might be due to the presence of an additional O in the –OCH3 group. However, the stability of HEMs materials is strongly dependent on their molecular structure and the bond lengths of the chemical substituents present in the phenyl ring.
The reported energetic 1,2,4-triazoles compounds can either be used as an explosive or propellants. We know that explosives and propellants generate supersonic and subsonic reaction waves, respectively, which propagate with the speed of several km s−1, inside the matter, accompanied by hot gases. These processes are known as detonation and deflagration.22 The thermal stability and efficiency of these compounds as rocket fuel are ascertained on the basis of strength of the PA signals produced due to the absorption of released NO2 molecules during the process of decomposition. In the present case, the –NH2 group increases the efficiency of the compound. The TG-DTA graph provides the information about the melting and decomposition temperatures and the residual weights of the compounds. The PA study also helps to differentiate the compounds based on their PA spectra produced due to absorption of 532 nm wavelength.
2. Theory and experimental details
The wave equation of sound pressure for the inhomogeneous medium in the lossless cylindrical resonator can be illustrated by23–26
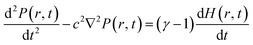 | (1) |
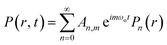 | (2) |
The dimensionless eigen modes distribution of the cylindrical resonator is the solution of the homogeneous wave equations, which can be expressed as follows:
| Pn(r, t) = Pn(r)eiωnt | (3) |
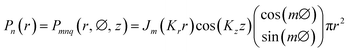 | (4) |
The amplitude (An) of the PA signal for the pulsed laser is proportional to the deposited heat density, which directly depends on the input laser power (E)27,28
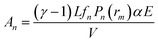 | (5) |
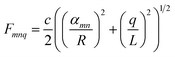 | (6) |
The experimental design is shown in Fig. 2. A Q-switched Nd:YAG laser (Model Spit, Germany) of wavelength 532 nm, pulse duration 7 ns and repetition rate 10 Hz was used to excite the vapor of the compounds in a cylindrical PA cavity. The laser beam diameter was adjusted to 6 mm using the aperture and allowed to pass through the centre of the PA cavity. The generated PA signal was detected by a pre-polarized microphone of responsivity 50 mV Pa−1 (BSWA, China), which was housed in the centre of the PA cell. The output signal of the microphone was fed into the preamplifier coupled to a 200 MHz oscilloscope (Tektronix, U.S.A.). The analysis was carried out using a data acquisition program, which was developed using LabView software.
A small quantity (∼1 mg) of solid compound was placed in a specially designed heating system, which facilitates the controlled pyrolysis between 30 and 350 °C. The entire system was evacuated up to 10−2 Torr using a rotary vacuum pump. The released vapor of the solid compound at the required temperature was introduced into the PA cell for recording of PA spectrum at the desired incident laser energy (Ein) and data acquisition time (t). The energy of the incident laser pulse was measured by a power meter (EPM2000, Coherent).
Thermogravimetric-differential thermal analysis (TG-DTA) was carried out using a TA instrument (Model no. Q600DT). The TG-DTA analysis was conducted with initial weights of 1.589 mg of P-Me-DNPT, 1.002 mg of P-OMe-DNPT, and 3.254 mg of P-NH2-DNPT. The solid compound was introduced into an alumina crucible and heated between 25 and 400 °C under a nitrogen gas atmosphere (flow rate of 100 cm3 min−1), which works as the purge and protective gas. An empty alumina crucible was used as reference. Non-isothermal TGA runs were conducted between 25 and 400 °C in a nitrogen atmosphere with purge rate of 10 °C min−1. In addition, infrared spectra of KBr pellets of these compounds were recorded using a Perkin-Elmer IR spectrometer between 400–4000 cm−1 range.
3. Results and discussion
3.1. IR spectra of the compounds
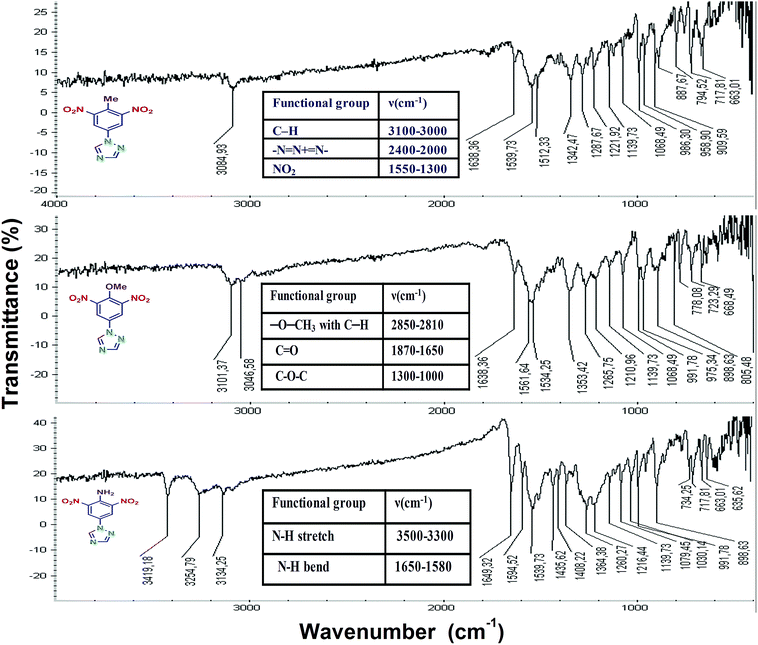
Fig. 3(a)–(c) show the IR spectra of P-Me-DNPT, P-OMe-DNPT, and P-NH2-DNPT, respectively. Inset tables of Fig. 3 show the structural positions of principal functional groups present in the compounds. The absorption peaks of the amino group are located at 3419.18, 3254.79 and 1649.32 cm−1. The strongest absorption peaks of C–O–C, –NO2, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+
N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N−, –OCH3 and C–H are observed at 1300–1000 cm−1, 1550–1300 cm−1, 2400–2000 cm−1 and 2850–2810 cm−1. The recorded FTIR spectra therefore confirm the presence of different types of functional groups in the reported compounds.
N−, –OCH3 and C–H are observed at 1300–1000 cm−1, 1550–1300 cm−1, 2400–2000 cm−1 and 2850–2810 cm−1. The recorded FTIR spectra therefore confirm the presence of different types of functional groups in the reported compounds.
3.2. Thermal PA fingerprint spectra of the compounds
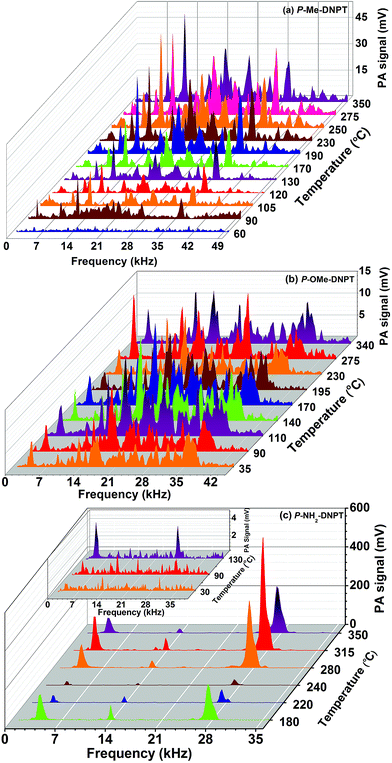
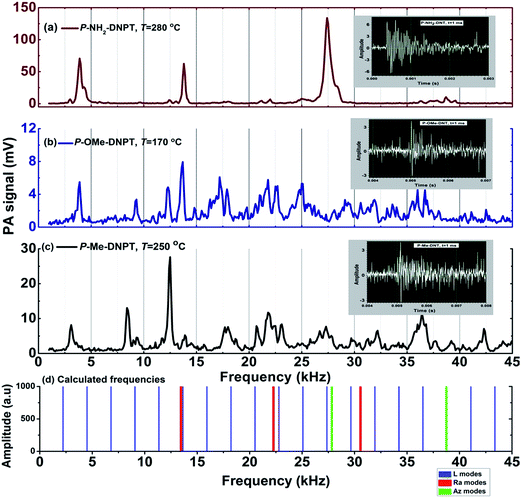
Fig. 4(a)–(c) show the thermal PA spectra of P-Me-DNPT, P-OMe-DNPT and P-NH2-DNPT, respectively. The PA spectra were obtained at incident laser energy of Ein = 8.63 mJ and data acquisition time of t = 0.5 ms.Fig. 4(a) shows that P-Me-DNPT does not provide any PA signal up to 60 °C. The first PA signal is observed at 90 °C, which is treated as an initial point of thermal decomposition. The PA spectra also show some sharp, intense acoustic modes located at 12.4 and 36.4 kHz. In addition, the other excited acoustic modes appear in the cluster form and occupy the range between 16 and 35 kHz. The excited acoustic modes of the PA cavity at Td = 250 °C are present at 3.2, 8.4, 12.4, 18, 20.8, 22, 23.2, 27.4, 32.2, 36.4, 42.2 and 44.2 kHz. The PA mode present at 12.4 kHz is one of the predominant acoustic modes of the cavity and has intensity of the order of 54.11 mV. It is observed that acoustic modes of the thermal PA spectra of the compounds have ±200 Hz variation with respect to their central frequency. The background noise signal of the system is of the order of 0.05 mV.
Fig. 4(b) shows the thermal PA spectra of P-OMe-DNPT. Though its melting temperature (Tm) is 97.92 °C, the process of dissociation is initiated at 30 °C. Therefore, it is once again confirmed that the NO2 molecules are released long before the melting temperature of the HEMs compounds.29,30 The majority of acoustic modes exhibit cluster behavior and have broad profiles due to the change in the density of the released vapor. The sharp, intense peaks are only located at 3.8 and 13.8 kHz between 30 and 350 °C range. However, the other higher intensity acoustic modes are present at 17.2, 22 and 35.8 kHz, respectively. It is observed that almost all acoustic modes of PA spectra are excited simultaneously and possess identical intensities. The PA spectra of these compounds are generated due to absorption of incident laser radiation by NO2, and presence of other gaseous molecules (released due to the methoxy group) leads to a change in the density of the cell medium, which affects the velocity of acoustic pressure waves. Consequently, the profile of acoustic modes shows a broadening effect along with a shift in the frequency with respect to the central frequency. The predominant order of excited acoustic modes gradually changes their position with respect to vapor temperature.
Fig. 4(c) shows the thermal PA spectra of P-NH2-DNPT. The compound shows two weak and one strong mode at 130 °C, which are present at 4.0, 13.8 and 27.4 kHz. This compound releases more NO2 molecules compared to the other compounds. Consequently, the PA spectrum has strong and sharp acoustic peaks. The maximum PA signal is obtained at 315 °C and the excited acoustic modes are located at 4, 12.4, 13.8, 27.4, 37.6, 38.6 and 39.11 kHz. During the experiment, thermally released reddish-brown colored vapor was observed in the heating flask, which clearly indicates that the vapor contains a high quantity of NO2 compared to other gaseous molecules.
Fig. 5(a)–(c) show the PA fingerprint spectra of the compounds. Inset figures show the corresponding time domain signals. The PA spectra were obtained at 250, 170 and 280 °C at t = 1 ms for P-Me-DNPT, P-OMe-DNPT and P-NH2-DNPT, respectively. Fig. 5(d) shows the total number of 24 calculated eigenmodes which comprise 19-longitudinal, 3-radial and 2-azimuthal modes of the PA cavity, which occupy the frequencies between 0 and 45 kHz. The first q, n and m mode frequencies are located at 2.28, 13.4 and 27.89 kHz, respectively. The calculated values of the sixth and twelfth longitudinal modes are almost equal to the first radial and azimuthal modes, respectively. It is inferred that these common eigenmodes are the strongest excited acoustic modes of the PA spectra of the samples, and clearly observed in the PA spectrum of P-NH2-DNPT. The sixth longitudinal and first radial modes are equally occupied the position at 13.8 kHz. Similarly, the twelfth longitudinal mode coincides with the first azimuthal mode and present at 27.4 kHz. The PA cavity has maximum numbers of even order longitudinal modes, and the strength of the PA signal and order of the excited acoustic modes vary from compound to compound and are attributed to the change in the density of released gaseous molecules.
 | ||
| Fig. 5 PA fingerprint spectra at t = 1 ms of (a) P-NH2-DNPT (b) P-OMe-DNPT (c) P-Me-DNPT, and (d) calculated longitudinal, radial and azimuthal modes of the PA cavity. | ||
In case of the PA spectra of P-Me-DNPT and P-OMe-DNPT, the acoustic modes are well separated and have sharp peaks between 0 and 15 kHz, whereas low intensity acoustic modes appear in pairs between 15 and 45 kHz. This confirms that the higher order modes are forming clusters due to percentile change of NO2. However, PA spectra of P-NH2-DNPT has two kinks at 4.4 and 28.3 kHz. The PA spectrum of P-NH2-DNPT depicts the well separated sharp peaks as compared to other two compounds and confirms that P-NH2-DNPT releases a more quantity of NO2 as compared to other byproduct gases. The small kinks of the PA spectra shows the presence of other gaseous molecules released in low quantities.
In our previous report, we showed the thermal stability and acoustic fingerprints of several new compounds such as 1-(2,4-dinitrobenzyl)-1H-1,2,3-triazole (S1), 1-(3,5-dinitrobenzyl)-1H-1,2,3-triazole (S2), 1-(2-methoxy-3,5-dinitrobenzyl)-1H-1,2,3-triazole (S3) and 1-(4-methoxy-3,5-dinitrobenzyl)-1H-1,2,3-triazole (S4).31 The effect of the methoxy group in the PA spectra of S3 and S4 was observed in the form of pairs. Additional acoustic peaks were absent in the PA spectra of S1 and S2. Similarly, P-OMe-DNPT also possesses a pair of acoustic peaks. The variation in the density of gaseous fragments is due to the presence of the –OCH3 group; as a result, the intensity of the acoustic mode is comparatively lower than that of the remaining two compounds.
The thermally released gases such as oxygen (O) or CO from methoxy groups, might react with freely released NO2 molecules and lead to convert the NO2 into NO along with formation of other gases, such as O2 and CO2, using the following chemical route:32,33
| NO2 + O → NO + O2 | (7) |
| NO2 + CO → NO + CO2 | (8) |
3.3. Thermal stability of compounds
Fig. 6(a), (c) and (e) show the behavior of acoustic modes with respect to temperature, while Fig. 6(b), (d) and (f) show TG-DTA thermo graphs in terms of weight loss and heat flow for P-Me-DNPT, P-OMe-DNPT and P-NH2-DNPT, respectively. In the case of P-Me-DNPT, the first predominant acoustic mode at 12.4 kHz has the highest PA signal at Td, i.e. 250 °C, as shown in Fig. 6(a). The compound starts releasing NO2 at 90 °C and this temperature is treated as the first thermal zone for the release of NO2. However, the fixed intensity of acoustic modes shows the stability of the compound between 90 and 170 °C. The acoustic modes located at 8.5 and 22 kHz are also stable between 190 and 350 °C.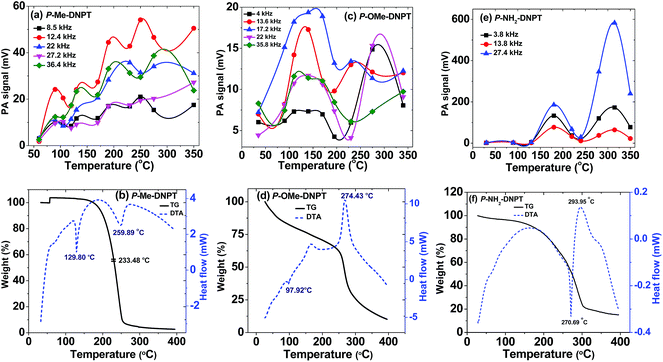 | ||
| Fig. 6 (a, c and e) Behavior of acoustic modes with respect to temperature, and (b, d and f) TG-DTA curves of P-Me-DNPT, P-OMe-DNPT, and P-NH2-DNPT respectively. | ||
Fig. 6(b) shows the heat flow curve of P-Me-DNPT, which confirms that the compound has a melting temperature at 129.80 °C and decomposition temperature at 259.89 °C. The weight loss curve shows that the compound is thermally stable up to 150 °C, and after crossing this temperature, the compound lost 95% of its total weight at 233.48 °C. Therefore, the strength of the PA signal and TG-DTA thermographs confirm that the P-Me-DNPT is thermally stable.
Fig. 6(c) shows the behavior of acoustic modes of P-OMe-DNPT with respect to temperature. The acoustic mode present at 17.2 kHz is one of the strongest PA modes between 90 and 195 °C. Similarly, modes present at 13.6 kHz show the highest PA peaks at 140 and 230 °C, whereas the mode at 17.2 kHz has higher peaks at 140 and 275 °C, respectively.
Fig. 6(d) shows the TG-DTA curves of P-OMe-DNPT, which has melting and decomposition temperatures at 97.92 and 274.43 °C, respectively. In addition, the heat flow curve shows the presence of a broad peak at 175 °C between 150 and 180 °C, which is also a decomposition point and correlated with strong PA signal due to the release of more NO2. The weight loss curve shows gradual decay in the weight of the compound between 25 and 275 °C. The rate of decomposition was accelerated after crossing 275 °C. This is also reflected in the strength of the PA signal of the acoustic modes present at 4, 22 and 35.8 kHz, which show stable behavior up to 275 °C.
Fig. 6(e) shows the behavior of three excited acoustic modes present at 3.8, 13.8 and 27.4 kHz, respectively. They show similar excitation behavior and intensities of the PA spectra shows two major peaks at 180 and 315 °C, respectively. The compound P-NH2-DNPT is thermally stable up to 130 °C. The heat flow curve of TG-DTA in Fig. 6(f) shows that the process of melting is initiated at 180 °C and is completed at 270 °C, which is followed by decomposition at 293 °C. The weight loss curve shows that the compound is thermally stable up to 150 °C, after which it loses around 70% of its total weight between 150 and 300 °C. However, the signature peak of the PA signal appears at 130 °C, which shows rapid growth up to 180 °C. The PA signal shows two additional peaks at 315 °C and 280 °C. The results obtained from the PA technique are in excellent agreement with the TG-DTA analysis. The P-NH2-DNPT has an additional N (due to –NH2), which increases the density of the compound (1.66 g cm−3) as compared to the other compounds and leads to the release of more quantity of NO2 than other gaseous molecules. Therefore, the synthesis of nitrogen (N)-rich, green energetic materials could be potential rocket fuels.2,3
The heat of decomposition (ΔH) depends on the overall decomposition and type of byproducts formed. The ring breaking reactions in the thermal decomposition process are either exothermic or endothermic in nature. The endothermic peaks represent the solid–solid phase-transition points that release condensed water. In the case of P-Me-DNPT, at the decomposition temperature (259 °C), the heat flow curve shows an endothermic peak (Fig. 6(b)), whereas the weight loss curve indicates that almost 95% weight is lost between 150 and 260 °C. This shows that the molecule starts decomposing at 259 °C, by releasing different types of gaseous byproducts along with condensed water (which is due to lack of high nitrogen content). Consequently, it shows an endothermic peak for P-Me-DNPT.34 However, P-OMe-DNPT and P-NH2-DNPT are oxygen- and nitrogen-rich compounds; as a result, at decomposition temperatures, the ring-breaking reactions appear in form of exothermic peaks (due to oxidation of nitrogen), as shown in Fig. 6(d) and (f), respectively. P-OMe-DNPT shows two exothermic peaks at 150 °C and 274.43 °C, respectively. The first exothermic peak is due to the breaking of principal functional groups attached to the ring, whereas the second one is due to a concerted ring breaking mechanism and indicates the completion of the total decomposition process.
3.4. Effect of incident laser energy (Ein)
Fig. 7(a)–(f) shows the PA spectra of 1,2,4-triazole compounds and the corresponding behavior of the acoustic modes with respect to incident laser energy. The thermal PA spectra of the compound was obtained at Ein = 8.63 mJ. We studied the effect of incident laser energy on the PA signal after crossing the Td, and recorded the threshold value of incident laser energy for the generation of PA signal.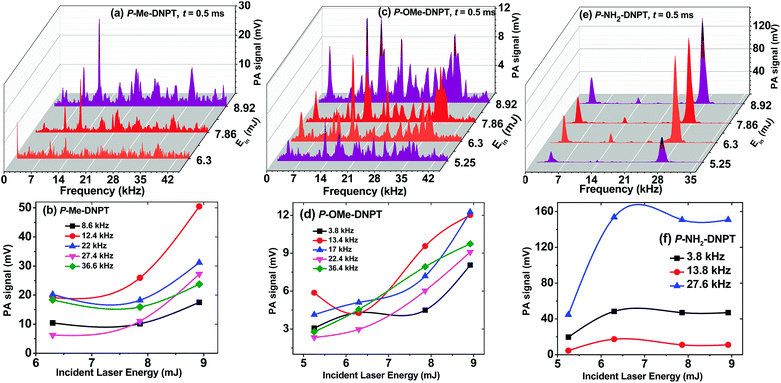 | ||
| Fig. 7 (a, c and e) PA spectra, and (b, d and f) behavior of acoustic modes of the compounds with respect to incident laser energy for P-Me-DNPT, P-OMe-DNPT, and P-NH2-DNPT respectively. | ||
The PA spectrum of P-Me-DNPT at Ein = 7.86 mJ shows well-distinguished and sharp acoustic modes as compared to 6.3 mJ. In the case of P-OMe-DNPT, 5.25 mJ energy is sufficient to generate the strong PA signal, which confirms that it releases a more quantity of gaseous products than the P-Me-DNPT. Thus, the experimental findings support that the release mechanism of NO2, along with other byproduct gaseous molecules, is purely dependent on the structures of the compounds and location of functional groups at the para position of the phenyl ring of 1,2,4-triazoles. However, the compound P-NH2-DNPT initiates the release of NO2 after crossing the 130 °C, which is also reflected in the PA signal. Therefore, P-NH2-DNPT needs lower incident laser energy to generate the strong PA signal.
The PA results also show that the phenyl series of 1,2,4-triazoles require high incident laser energy as compared to the previously reported benzyl series of 1,2,3-triazoles.31,35 Since the phenyl series of compounds have direct bonding between two aromatic rings, i.e. the 1,2,4-triazole moiety and the phenyl ring, this series of compounds release low quantities of gaseous products. However, in the case of the benzyl series, the 1,2,3-triazole moiety is connected to the phenyl ring through the –CH2 group. Thus, during the thermal decomposition process, the benzyl series of 1,2,3-triazoles release more gaseous fragments than of the phenyl series of 1,2,4-triazoles; therefore, they require lower incident laser energy to generate a strong PA signal.
3.5. Effect of data acquisition time (t)
The acoustic wave activation time is controlled by the data acquisition time through the oscilloscope. The PA spectra, at different data acquisition times, provide detailed information about the behavior of acoustic modes with respect to the different time frames. Fig. 8(a)–(f) show the PA spectra and decay behavior of acoustic modes with respect to data acquisition time for P-Me-DNPT, P-OMe-DNPT, and P-NH2-DNPT, respectively. The PA spectra are obtained at the decomposition temperatures and Ein = 8.63 mJ. The excited acoustic modes located at 22, 13.8 and 4 kHz have corresponding decay times of the order of 0.43, 0.26, and 0.25 ms, respectively. For P-NH2-DNPT, the decay times of the acoustic modes are lower than for the other compounds. This clearly indicates that the compounds that have high concentrations of NO2 molecules possess lower decay times due to short collision times.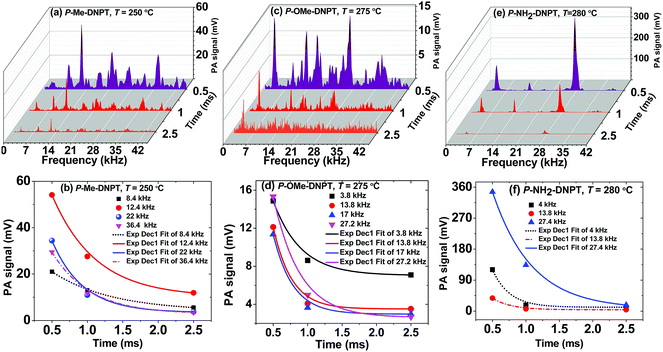 | ||
| Fig. 8 (a, c and e) PA spectra, and (b, d, and f) behavior of acoustic modes with data acquisition time for P-Me-DNPT, P-OMe-DNPT, and P-NH2-DNPT respectively. | ||
It was also observed that at t = 2.5 ms, the magnitude of the excited acoustic modes of P-OMe-DNPT become minimum and provide weak PA signals. Therefore, according to the kinetic theory of gases, the selection of lower time scales i.e. t ≤ 1 ms is most suitable for recording the PA signal for the trace level detection of gaseous products. Since all the excited acoustic modes show exponential decay behavior with different decay times, the selection of t = 0.5 ms provides the strong PA signal of high resolution as compared to the other time scales.
3.6. Bond breaking mechanism and scaling the efficiency of the compounds as rocket fuel
The sequence of the bond breaking mechanisms of the major functional groups from the solid compounds during the pyrolysis process is explained in terms of their bond lengths. The bond lengths of the compounds were calculated by optimizing the structures in the Gaussian 03 program using B3PW91 functional with 6-31G(d,p) basis set.21 The bond lengths of the principal functional groups are listed in Table 1.| Functional groups | P-Me-DNPT | P-OMe-DNPT | P-NH2-DNPT |
|---|---|---|---|
| Cphenyl–Ntriazole | 1.407 Å | 1.412 Å | 1.410 Å |
| Cphenyl – para group | 1.503 Å | 1.345 Å | 1.330 Å |
| (C–CH3) | (C–OCH3) | (C–NH2) | |
| –C–NO2 | 1.474 Å | 1.476 Å | 1.450 Å |
| Strength of PA signal | 54.11 mV | 19.36 mV | 582.99 mV |
In the case of P-Me-DNPT, the methyl group is present at the para position of the phenyl ring with a sigma bond having a bond length of the order of 1.503 Å. In addition, two NO2 groups are attached to the 3,5(meta)-positions of the phenyl ring via sigma bonds having a bond length of the order of 1.474 Å. Since the bond length of the para position methyl group is greater than those of the nitro groups, the supplied heat energy during pyrolysis was first used to cleave the methyl group followed by the nitro group and then triazole group. As a result, a smaller quantity of NO2 molecules were released. For P-NH2-DNPT, the bond length between the para position amino group and the phenyl ring is 1.330 Å, and the bond length between meta nitro groups and the phenyl ring is 1.450 Å. In this case, the supplied heat energy at initial level is utilized to cleave the nitro groups from the phenyl ring rather than amino group. Consequently, a high yield of NO2 molecules is released, which leads to the generation of the strongest PA signal, of the order of 582.99 mV. Similarly, in the case of P-OMe-DNPT, the bond length between the para methoxy group and phenyl ring is 1.345 Å, and the bond length between the nitro group and the phenyl ring is of the order of 1.476 Å. Therefore, the decomposition process is initiated with release of NO2 molecules and followed by methoxy group fragmentation. Furthermore, the –OCH3 group is fragmented into O, CO, H2, and H2O. It appears that the concentration of any of these fragments is equally high along with NO2 in the gas mixture, which has no absorption in 532 nm range. Therefore, the acoustic modes possess pairs of peaks and have low strength PA signals for P-OMe-DNPT, even though P-OMe-DNPT initially releases a high quantity of NO2 along with other gaseous molecules. The strength of the PA signal is only monitored in terms of NO2 concentration, whereas the presence of other gases leads to broadening of the profile of acoustic modes along with the shift from actual calculated values of modes.
The rupture of single bond (C–N) molecules requires lower energy than the double bond (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) molecules because the double bonded molecules have higher bond strengths. Therefore, the process of decomposition of the reported 1,2,4-triazoles is completed in two steps. In the first step, the triazole moiety is separated out from the phenyl ring, and the final step occurs with concerted ring breaking. The aromatic ring, such as the triazole moiety, requires higher energy than the aliphatic groups, such as –CH3, –OCH3 and –NH2, for separation from the ring. The entire molecules along with their principal functional groups decompose easily at high temperatures. However, during the pyrolysis of HEMs compounds between 30 and 350 °C, they release their fragments and higher concentrations of gaseous products at Tm, Td, and after crossing the Td, which shows the thermal stability of the compounds. The expected order of cleaved functional groups from the ring during the process of decomposition with respect to the supplied heat energy is listed in Table 2.
N) molecules because the double bonded molecules have higher bond strengths. Therefore, the process of decomposition of the reported 1,2,4-triazoles is completed in two steps. In the first step, the triazole moiety is separated out from the phenyl ring, and the final step occurs with concerted ring breaking. The aromatic ring, such as the triazole moiety, requires higher energy than the aliphatic groups, such as –CH3, –OCH3 and –NH2, for separation from the ring. The entire molecules along with their principal functional groups decompose easily at high temperatures. However, during the pyrolysis of HEMs compounds between 30 and 350 °C, they release their fragments and higher concentrations of gaseous products at Tm, Td, and after crossing the Td, which shows the thermal stability of the compounds. The expected order of cleaved functional groups from the ring during the process of decomposition with respect to the supplied heat energy is listed in Table 2.
| Compound | Order |
|---|---|
| P-Me-DNPT | –CH3, –NO2, –triazole |
| P-OMe-DNPT | –NO2, –triazole, –OCH3 |
| P-NH2-DNPT | –NO2, –triazole, –NH2 |
The decomposition mechanism might be more complex and it may involve bond rearrangements and isomerizations before fragmentation or decomposition. However, the order of cleaved functional groups can be confirmed based on the PA spectra of the compounds in terms of their excited acoustic modes and signal intensities.
The estimated percentile residual quantities of compounds from the weight loss curves at 400 °C are as follows. P-Me-DNPT: 3% (initial weight (Iw): 1.589 mg), P-OMe-DNPT: 10% (Iw: 1.002 mg) and P-NH2-DNPT: 15% (Iw: 3.254 mg). The highest PA signals obtained for these compounds at t = 0.5 ms are 54.11, 19.36 and 582.99 mV, respectively. For t = 1 ms, the strength of the signals are 30.08, 9.7 and 36.12 mV, respectively. Even though the initial weight of P-OMe-DNPT was less, it showed comparatively high residual weight and lower PA signal strength than the other two compounds. On the basis of the residual weight from TG-DTA and the obtained strengths of the PA signals, the efficiencies of these compounds as a rocket fuel is in the sequence of P-NH2-DNPT > P-Me-DNPT > P-OMe-DNPT. A similar order is applicable to the explosive properties of these compounds. The efficiencies of these compounds as a rocket fuel were ascertained on the basis of released quantities of NO2 molecules in terms of the strength of PA signals. This is because NO2 is identified as a freely released gas during the pyrolysis of HEMs and is treated as a thermal marker.29–31
3.7. Comparison between GC-MS and PA techniques
The gas chromatography mass spectroscopy (GC-MS) is a well known analytical technique, which is used for solids, liquids and gases. It works on the principle of column separation for which the solid sample is required to be dissolved in a particular solvent. Moreover, the entire process starts at above 200 °C in the oven. The solid compound is heated up to the required temperature and introduced into the oven along with the injector (N2 or He) after measuring the individual concentrations of each gas composition based on their column condition and the retention time. However, the PA pyrolysis technique is simple and non destructive in nature; it does not require sample preparation and needs very small quantity of solid samples. Herein, laser excitation wavelength is selected according to the absorption characteristics of released gas (for our case NO2) from the solid compound. In addition, it does not require any purge gas. The PA signal is produced due to non radiative transition, which is detected by a prepolarized microphone. It is one of the most sensitive detection techniques and has low level detection limit of the order of ppb. The GC-MS requires minimum vapor pressure of the order of 10 Torr, whereas the PA technique needs vapor pressure even less than 1 Torr. Furthermore, the controlled pyrolysis of the compounds help us to monitor the release of NO2 gas at different temperatures. Apart from NO2, other gaseous components can also be identified by the selection of tunable laser wavelengths.Unlike GC-MS, the present form of the PA technique is based on 532 nm wavelength and unable to monitor the individual concentrations of byproduct gaseous molecules released from HEMs during the thermal decomposition process. However, we can monitor the release of NO2 below the melting temperature to study the thermal stability of the compound. Moreover, the change in the density of the vapor is observed in terms of a shift in the frequency of acoustic modes.
4. Conclusions
We successfully obtained the thermal PA spectra of the newly synthesized 1,2,4-triazoles. The role of the bond breaking mechanism of the principal functional groups during the thermal decomposition process between 30 and 350 °C has been examined in terms of their bond lengths. The thermal stabilities of these compounds are explained based on the strength of the PA signals and TG-DTA analysis. In addition, the efficiencies of these compounds as a rocket fuel for military applications and explosives have been investigated and found to be in the order P-NH2-DNPT > P-Me-DNPT > P-OMe-DNPT. The effects of incident laser energy and data acquisition time on the PA signal of the compounds were also studied. The study reveals that the compound P-NH2-DNPT requires less incident laser energy compared to the other compounds. In the case of the reported phenyl series of 1,2,4-triazoles, t ≤ 1 ms is the suitable data acquisition time to obtain their thermal photoacoustic fingerprint spectra.Acknowledgements
The authors gratefully acknowledge the D.R.D.O., Ministry of Defence, Govt. of India, India, for financial support. Our thanks are due to Dr K. V. Rao, Director, ACRHEM, University of Hyderabad, for moral encouragement and keen interest.References
- Q. Wu, W. Zhu and H. Xiao, RSC Adv., 2014, 4, 53000–53009 RSC.
- D. Srinivas, V. D. Ghule and K. Muralidharan, RSC Adv., 2014, 4, 7041–7051 RSC.
- A. S. Kumar, V. D. Ghule, S. Subrahmanyam and A. K. Sahoo, Chemistry, 2013, 19, 509–518 CrossRef CAS PubMed.
- Y. Zhang, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2013, 1, 585–593 CAS.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418 RSC.
- T. M. Klapötke and C. M. Sabaté, Chem. Mater., 2008, 20, 3629–3637 CrossRef.
- H. S. Jadhav, M. B. Talawar, R. Sivabalan, D. D. Dhavale, S. N. Asthana and V. N. Krishnamurthy, J. Hazard. Mater., 2007, 143, 192–197 CrossRef CAS PubMed.
- P. Zhang, S. J. Klippenstein, L. B. Harding, H. Sun and C. K. Law, RSC Adv., 2014, 4, 62951–62964 RSC.
- T. M. Klapo, P. Mayer, A. Schulz and J. J. Weigand, J. Am. Chem. Soc., 2005, 127, 2032–2033 CrossRef PubMed.
- X. Liu, Q. Yang, Z. Su, S. Chen, G. Xie, Q. Wei and S. Gao, RSC Adv., 2014, 4, 16087–16093 RSC.
- V. Kraft, W. Weber, M. Grutzke, M. Winter and S. Nowak, RSC Adv., 2015, 5, 80150–80157 RSC.
- J. Cheng, R. Zhang, Z. Liu, L. Li, F. Zhao and S. Xu, RSC Adv., 2015, 5, 50278–50288 RSC.
- R. Turcotte, M. Vachon, Q. S. M. Kwok, R. Wang and D. E. G. Jones, Thermochim. Acta, 2005, 433, 105–115 CrossRef CAS.
- M. F. Foltz, C. L. Coon, F. Garcia and A. L. Nichols III, Propellants, Explos., Pyrotech., 1994, 19, 133–144 CrossRef CAS.
- D. E. G. Jones, P. D. Lightfoot, R. C. Fouchard, Q. Kwok, A. M. Turcotte and W. Ridley, Thermochim. Acta, 2002, 384, 57–69 CrossRef CAS.
- F. J. M. Harren, G. Cotti, J. Oomens and S. L. T. Hekkert, Encycl. Anal. Chem., 2000, pp. 2203–2226 Search PubMed.
- S. Schäfer, M. Mashni, J. Sneider and A. Mikl, Appl. Phys. B, 1998, 66, 511–516 CrossRef.
- M. S. Churio, M. a. Brusa, M. a. Grela, S. G. Bertolotti and C. M. Previtali, Phys. Chem. Chem. Phys., 2003, 5, 902 RSC.
- C. L. Spencer, V. Watson and M. Hippler, Analyst, 2012, 137, 1384 RSC.
- J. W. Lu, J. M. Flores, A. Lavi, A. Abo-Riziq and Y. Rudich, Phys. Chem. Chem. Phys., 2011, 13, 6484 RSC.
- N. Kommu, V. D. Ghule, A. S. Kumar and A. K. Sahoo, Chem.–Asian J., 2014, 9, 166–178 CrossRef CAS PubMed.
- J. A. Conkling, Propellants, Scientific American, 2nd edn, 1996 Search PubMed.
- A. Miklós, P. Hess and Z. Bozóki, Rev. Sci. Instrum., 2001, 72, 1937–1955 CrossRef.
- F. Yehya and A. K. Chaudhary, J. Mod. Phys., 2011, 02, 200–209 CrossRef.
- R. Bartlome, M. Kaučikas and M. W. Sigrist, Appl. Phys. B: Lasers Opt., 2009, 96, 561–566 CrossRef CAS.
- F. Yehya and A. K. Chaudhary, Opt. Commun., 2014, 312, 16–22 CrossRef CAS.
- L. Y. Hao, J. X. Han, Q. Shi, J. H. Zhang, J. J. Zheng and Q. S. Zhu, Rev. Sci. Instrum., 2014, 1975, 1–7 Search PubMed.
- L. Krämer, Z. Bozoki and R. Niessner, Anal. Sci., 2001, 17, 563–566 Search PubMed.
- F. Yehya and A. K. Chaudhary, Sens. Actuators, B, 2013, 178, 324–330 CrossRef CAS.
- F. Yehya and A. K. Chaudhary, Appl. Phys. B, 2012, 110, 15–22 CrossRef.
- K. S. Rao, F. Yehya, A. K. Chaudhary, A. S. Kumar and A. K. Sahoo, J. Anal. Appl. Pyrolysis, 2014, 109, 132–139 CrossRef CAS.
- P. J. Crutzen and M. Oppenheimer, Clim. Change, 2008, 89, 143–154 CrossRef CAS.
- A. Burcat and A. Lifshitz, J. Phys. Chem., 1970, 74, 263–268 CrossRef CAS.
- A. S. Kumar, N. Kommu, V. D. Ghule and A. K. Sahoo, J. Mater. Chem. A, 2014, 2, 7917 CAS.
- K. S. Rao and A. K. Chaudhary, Thermochim. Acta, 2015, 614, 149–156 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |