Terpyridinyl dibenzo[b,d]furan and dibenzo[b,d]thiophene based tetrameric bismetallo-macrocycles†
Tun Wu‡
a,
Guo Yuan‡a,
Die Liua,
Meng Wanga,
Yiming Lia,
Mingzhao Chena,
Hongwen Taob and
Pingshan Wang*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha-410083, Hunan, China. E-mail: chemwps@csu.edu.cn; Fax: +86-731-88879616; Tel: +86-731-88879616
bCollege of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan-411201, Hunan, China
First published on 5th January 2016
Abstract
Novel dibenzo[b,d]thiophene and dibenzo[b,d]furan-based bisterpyridine ligands have been synthesized and used to create unique metallomacrocycles. Direct self-assembly between the bisterpyridine ligands and Fe2+ led to a mixture containing multiple homo-metallomacrocycles. However, utilizing the robust Tpy(TpyRu2+Tpy)Tpy ligands, the metallo-organic ligands, coordination with Fe2+ resulted in the pure tetrameric bismetallo-macrocycles. Structures were characterized by 1H-NMR, COSY-NMR, DOSY-NMR, ESI-TOF-MS and UV/vis spectra.
Over the past two decades, the design and construction of highly ordered, supramolecular architectures have attracted considerable attention, especially metallic macrocycles.1 The innovative works of Lehn,2–4 Stang,5,6 Fujita,7 Newkome7 and many others8–11 in the field of self-assembly have shown that understanding the nature of the intermediate is an important tool in determining the outcome of the final supramolecular structure. In terms of a coordination-driven approach to supramolecular structures, there is growing concern with the 2,2′:6′,2′′-terpyridine ligands as a building block due to its strong binding with many transition metal ions,12–14 metallomacrocycles with trigonal-,15–17 quadrilateral-,18–21 pentagonal-,22,23 and hexagonal-shaped24–27 motifs have been achieved by utilizing polyterpyridine-based metallo-ligand self-assembly methods. However, terpyridine-based organic ligand is more flexible than expected which could lead to the formation of multicomponent mixture.27
Herein, we describe an approach of synthesizing a unique set of macrocycles employing two novel bisterpyridine ligands (L1 and L2) based on dibenzo[b,d]thiophene and dibenzo[b,d]furan units. The stretch angles of L1 and L2 are approximately 79.5° and 90°, respectively, and this becomes of apparent significance upon complexation.28 The ability of L1 to self-assemble with Fe2+ generated mixed macrocycles with metallocycles ranging from triangular to hexameric in nature; however, coordination of L2, due to its close 90° bend angle, with Fe2+ yielded only a tetrameric cycle and small amounts of a pentameric cycle (Scheme 1). In order to construct single quantities of metallo-macrocycles, a step-wise approach was employed. First, a half-square bracket metallo-organic ligand (D3 and D4, Scheme 2) with two uncomplexed free terpyridines was constructed; thereafter, when coordinated with Fe2+, nearly quantitative yields of two dinuclear tetrameric cycles were obtained.
Synthesis of the key organic building blocks (L1 and L2) began by altering commercially available dibenzo[b,d]thiophene and dibenzo[b,d]furan to give 2,8-dibromodibenzo[b,d]thiophene and 2,8-dibromodibenzo[b,d]furan.29 Subsequent reaction with (4-([2,2′:6′,2′′-terpyridin]-4′-yl)phenyl)boronic acid21 via Suzuki-coupling yielded the desired ligands (see ESI, Scheme S1†). The ligands were structurally confirmed by 1H-NMR (Fig. S1 and S2†), 13C-NMR (Fig. S11 and S12†) and LC-MS (Fig. S15 and S16†).
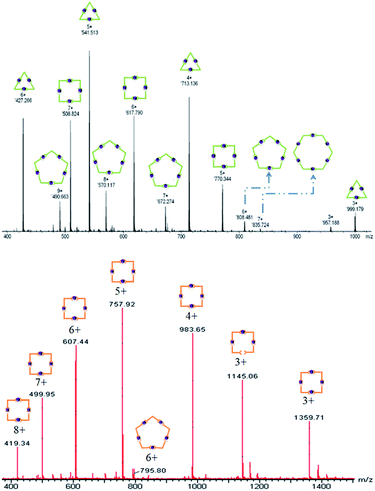
L1 reacted with 1 equiv. of FeCl2 in a mixed solvent (CHCl3/MeOH = 1/1) to produce an inseparable metallo-macrocyclic mixture (Scheme 1 and Fig. 1). The mixed structures were confirmed by 1H-NMR, (Fig. 1, top) showing the most characteristic peak of tpyH3′,5′ revealed in multiple peaks. In addition, ESI-TOF-MS indicated the multi-macrocycles (L1Fe)x by revealing the metallo-trimer (m/z = 427.266, 541.513, 713.136 and 999.179 corresponding to [(L1Fe)3-nPF6]n+, n = 3–6), metallo-tetramer (m/z = 508.824, 617.79 and 779.344 corresponding to [(L1Fe)4-nPF6]n+, n = 5–7), metallo-pentamer (m/z = 490.663, 570.117, 672.274 and 808.481 corresponding to [(L1Fe)5-nPF6]n+, n = 6–9), and trace amounts of the metallo-hexamer (m/z = 835.724 corresponding to [(L1Fe)6-7PF6]7+) (Fig. 2, top).
 | ||
| Fig. 1 1H-NMR of metallo-macrocycles [(L1Fe)x (top) and (L2Fe)x (bottom)] from directly self-assembly with Fe2+. | ||
When treating L2 with 1 equiv. of FeCl2, 1H-NMR showed multiple peaks corresponding to the tpyH3′,5′ proving that the product also is a mixture, but more distinguishable than (L1Fe)x (Fig. 1, bottom). The ESI-TOF-MS spectrum confirmed a main product of the metallo-tetramer (m/z = 419.34, 499.95, 607.44, 757.92, 983.65 and 1359.71 corresponding to [(L2Fe)4-nPF6]n+, n = 3–8) and trace amounts of the metallo-pentamer (m/z = 795.80 corresponding to [(L2Fe)5-4PF6]6+) (Fig. 2, bottom). The most likely reason is the extended phenyl group in L1 increased the rotation and bending flexibility, leading to the diversity in the molecular coordination.21 In the results from L2, the angle was much closer to 90° which decreased the rotational freedom during the self-assembly process.
It has been reported that the [Tpy-Ru-Tpy]2+ structure21 is strong enough to obstruct scrambling of ligands. Thus, a step-wise coordination method was used for the design and synthesis of metallo-dimmers [(L1RuL1)Cl2] and [(L2RuL2)Cl2] (D3 and D4) from a one-pot reaction. 1H-NMR of dimer 3 (D3) exhibited two distinct resonances of tpyH3′,5′ at 9.29 ppm and 8.75 ppm with an integration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as expected. In addition, peaks at m/z = 566.5, 849.2 for [M − 2Cl− + H+]3+, [M − 2Cl−]2+ (ESI, Fig. S17†) also provided evidence for the formation of D3. In terms of D4, along with consistent 1H-NMR spectra, two distinct resonances of tpyH3′,5′ were at 9.54 ppm and 8.87 ppm with an integration ratio of 1
1 as expected. In addition, peaks at m/z = 566.5, 849.2 for [M − 2Cl− + H+]3+, [M − 2Cl−]2+ (ESI, Fig. S17†) also provided evidence for the formation of D3. In terms of D4, along with consistent 1H-NMR spectra, two distinct resonances of tpyH3′,5′ were at 9.54 ppm and 8.87 ppm with an integration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ESI-TOF-MS showed peaks at m/z = 417.1, 555.8 and 833.2 for [M − 2Cl−+2H+]4+, [M − 2Cl− + H+]3+ and [M − 2Cl−]2+ respectively (Fig. S18†).
1, ESI-TOF-MS showed peaks at m/z = 417.1, 555.8 and 833.2 for [M − 2Cl−+2H+]4+, [M − 2Cl− + H+]3+ and [M − 2Cl−]2+ respectively (Fig. S18†).
Subsequently, D3 and D4 were treated with FeCl2 in MeOH. After the simple counterion exchange with NH4PF6, two desired metallo-tetramers were obtained in nearly quantitative yield (Scheme 2). The 1H-NMR spectra for metallo-tetramer 5 (T5, Fig. 2), where the color denotes the respective metals, displayed two resonances of tpyH3′,5′ for each assignable proton, all integrating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In comparison to D3, protons at the 6,6′′ position of Tpy-B (purple) exhibited a dramatic shift from 8.71 to 7.29 ppm due to the electron shielding effect after complexation with Fe2+ (Fig. 3, Fig. S5 and S9†). 1H-NMR for metallocycle 6 (T6) presented a similar situation in which protons at the 6,6′′ position of Tpy-B (purple) shifted upfield from 8.79 ppm to 7.29 ppm (Fig. 4, S6 and S10†). The whole aromatic region displayed two resonances for each peak assignations proton with an integration ratio of 1
1. In comparison to D3, protons at the 6,6′′ position of Tpy-B (purple) exhibited a dramatic shift from 8.71 to 7.29 ppm due to the electron shielding effect after complexation with Fe2+ (Fig. 3, Fig. S5 and S9†). 1H-NMR for metallocycle 6 (T6) presented a similar situation in which protons at the 6,6′′ position of Tpy-B (purple) shifted upfield from 8.79 ppm to 7.29 ppm (Fig. 4, S6 and S10†). The whole aromatic region displayed two resonances for each peak assignations proton with an integration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All of the peak assignations were based on 2D COSY NMR.
1. All of the peak assignations were based on 2D COSY NMR.
 | ||
Fig. 3 Stacked 1H-NMR spectra of L1 (CDCl3), D3 (CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD = 1 CD3OD = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), T5 (CD3CN), where Ru2+ represented as the red balls, Fe2+ as the purple balls. 1), T5 (CD3CN), where Ru2+ represented as the red balls, Fe2+ as the purple balls. | ||
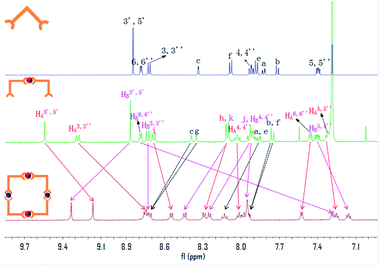 | ||
Fig. 4 Stacked 1H-NMR spectra of L2 (CDCl3), D4 (CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD = 1 CD3OD = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), T6 (CD3CN), where Ru2+ represented as the red balls, Fe2+ as the purple balls. 1), T6 (CD3CN), where Ru2+ represented as the red balls, Fe2+ as the purple balls. | ||
2D DOSY-NMR spectroscopy has frequently been utilized to determine the purity of the complexes due to its effective characterization for molecules having relatively larger molecular weights and for monitoring self-assembly processes by correlating chemical resonances to diffusion coefficients in solutions.30 T5 and T6 (Fig. 5) showed only one component in solution for each substance. The diffusion coefficients measured in CD3CN at 298 K are 2.796 × 10−10 m2 s−1 for T5, and 2.759 × 10−10 m2 s−1 for T6, respectively. Experimental hydrodynamic radius for T5 and T6 were calculated to be 2.13 nm and 2.16 nm via the Stoke–Einstein equation D = kBT/6πηrH (kB, Boltzman constant; T, absolute temperature; η = 0.367 mPa s, viscosity of CD3CN at 298 K).31
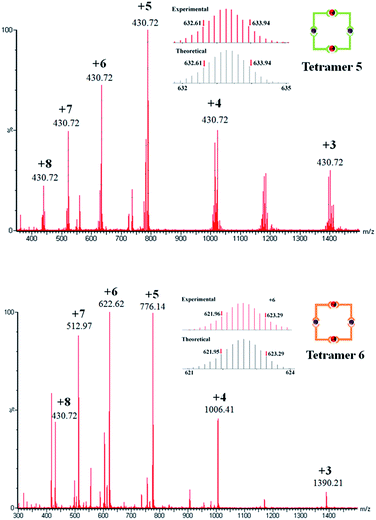
Further support for the formation of T5 was provided by ESI-TOF-MS signals for the multiply-charged species in charge states +8 (m/z 438.21), +7 (m/z 521.9673), +6 (m/z 633.2730), +5 (m/z 788.92), +4 (m/z 1022.39) and +3 (m/z 1401.22). The detected results and the isotope pattern of each peak agreed well with the corresponding theoretical distributions (Fig. 6 top, also see ESI†). In terms of T6, there are several unambiguous peaks for the multiply-charge states +8 (m/z 430.72), +7 (m/z 512.97), +6 (m/z 622.62), +5 (m/z 776.14) +4 (m/z 1006.41), +3 (m/z 1390.21). Likewise, slight differences between the detected isotope pattern and the corresponding theoretical distributions of each peak have been found (Fig. 6 bottom, also see ESI†).
UV-vis spectroscopy for the compounds was examined in MeCN. For D3, the major absorption band at λ = 310 nm originated from intra-ligand charge transfer in addition to the characteristic absorption band at λ = 496 nm indicating the MLCT of Ru2+ to terpyridine ligands. Further, T5 revealed two MLCT bands, λ = 494 and 572 nm, representing Ru2+ and Fe2+, respectively (Fig. 7 left, Fig. S22†). As well, the similar UV-vis spectra of D4 and T6 were illustrated in Fig. 7 (right, Fig. S23†). The electrochemical properties of complexes T5 and T6 were investigated by cyclic voltammetry (see ESI, Fig. S24†), all complexes illustrated one reversible oxidation couple of Ru2+/Ru3+ and the moderate onset oxidation potentials, which was determined to be 1.50 V (T5) and 1.70 V (T6) vs. Hg/Hg2Cl2, respectively. In addition, the reversible oxidation couple of Fe2+/Fe3+ were measured to be 0.73 V in T5 and 1.15 V in T6, respectively.
Conclusions
In conclusion, two dibenzo[b,d]thiophene and dibenzo[b,d]furan-based bisterpyridinyl ligands possessing uniquely restricted angles, have been synthesized and proven to self-assemble with transition metals to form two dinuclear tetrameric metallomacrocycles by means of a step-wise strategy. The structures of the two metallo-tetramers were confirmed by 1H-NMR, COSY-NMR, DOSY-NMR, ESI-TOF-MS and UV-vis. This study demonstrates that controlling the angled metallo-ligands is possible for the purpose of preparing pure square-shaped supramolecular metallomacrocycles. It provides an effective method to further probe the characteristics, structure and stability of [TpyRuTpy]2+ compounds in order to design and prepare Ru-based terpyridine complexes with enhanced photophysical properties.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21274165) and the Distinguished Professor Research Fund from Central South University of China. The authors gratefully acknowledge Dr Carol D. Shreiner for her professional consultation. Authors acknowledge the NMR measurements from The Modern Analysis and Testing Center of CSU.Notes and references
- J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995 RSC; U. S. Schubert, H. Hofmeier and G. R. Newkome, Modern Terpyridine Chemistry, Wiley-VCH, Weinheim, 2006 RSC; L. Cronin, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2004, 100, 323–383 RSC.
- M. Ruben, U. Ziener, J. M. Lehn, V. Ksenofontov, P. Gutlich and G. B. M. Vaughan, Chem.–Eur. J., 2005, 11, 84–100 CrossRef PubMed.
- M. Barboiu, G. Vaughan, R. Graff and J. M. Lehn, J. Am. Chem. Soc., 2003, 125, 10257–10265 CrossRef CAS PubMed.
- M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J. M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3644–3662 CrossRef CAS PubMed.
- S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS PubMed.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed.
- G. R. Newkome and C. N. Moorefield, Chem. Soc. Rev., 2015, 44, 3954–3967 RSC.
- L. R. MacGillivray and J. L. Atwood, Angew. Chem., Int. Ed., 1999, 38, 1018 CrossRef CAS.
- B. J. Holliday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022 CrossRef CAS; E. Baranoff, J. P. Collin, L. Flamigni and J. P. Sauvage, Chem. Soc. Rev., 2004, 33, 147 RSC; E. C. Constable, C. E. Housecroft, M. Neuburger, S. Schaffner and C. B. Smith, Dalton Trans., 2005, 13, 2259 RSC; J. M. Lehn, Chem. Soc. Rev., 2007, 36, 151 RSC; N. Giuseppone, J. L. Schmitt, L. Allouche and J. M. Lehn, Angew. Chem., Int. Ed. Engl., 2008, 47, 2235 CrossRef PubMed; R. S. Forgan, J. P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434 CrossRef PubMed; I. A. Riddell, Y. R. Hristova, J. K. Clegg, C. S. Wood, B. Breiner and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 2723 CrossRef PubMed; A. M. Castilla, W. J. Ramsay and J. R. Nitschke, Acc. Chem. Res., 2014, 47, 2063 CrossRef PubMed; P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef; M. Yoshizawa, K. Kumazawa and M. Fujita, J. Am. Chem. Soc., 2005, 127, 13456 CrossRef PubMed; P. Mobian, J.-M. Kern and J.-P. Sauvage, J. Am. Chem. Soc., 2003, 125, 2016 CrossRef PubMed; F. Ibukuro, T. Kusukawa and M. Fujita, J. Am. Chem. Soc., 1998, 120, 8561 CrossRef; A. E. V. Gorden, J. Xu, K. N. Raymond and P. Durbin, Chem. Rev., 2003, 103, 4207 CrossRef PubMed; T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang and K.-W. Chi, Acc. Chem. Res., 2013, 46, 2464 CrossRef PubMed; S. J. Cantrill, K. S. Chichak, A. J. Peters and J. F. Stoddart, Acc. Chem. Res., 2005, 38, 1 CrossRef PubMed; M. Barboiu, G. Vaughan, R. Graff and J.-M. Lehn, J. Am. Chem. Soc., 2003, 125, 10257 CrossRef PubMed; M. Fujita, N. Fujita, K. Ogura and K. Yamaguchi, Nature, 1999, 400, 52 CrossRef; M. Fujita, Chem. Soc. Rev., 1998, 27, 417 RSC; P. J. Stang, Chem.–Eur. J., 1998, 4, 19 CrossRef; R. Ziessel, Synthesis, 1999, 11, 1839 CrossRef; M. Wang, C. Wang, X. Q. Hao, J. Liu, X. Li, C. Xu, A. Lopez, L. Sun, M. P. Song, H. B. Yang and X. Li, J. Am. Chem. Soc., 2014, 136, 6664 CrossRef PubMed.
- S. J. Lee, A. Hu and W. Lin, J. Am. Chem. Soc., 2002, 124, 12948 CrossRef CAS PubMed.
- H. Jiang and W. Lin, J. Am. Chem. Soc., 2003, 125, 8084 CrossRef CAS PubMed.
- D. Armspach, M. Cattalini, E. C. Constable, C. E. Housecroft and D. Phillips, Chem. Commun., 1996, 1823 RSC.
- G. R. Newkome, E. He, L. A. Godinez and G. R. Baker, J. Am. Chem. Soc., 2000, 122, 9993 CrossRef CAS.
- A. Wild, A. Winter, F. Schlutter and U. S. Schubert, Chem. Soc. Rev., 2011, 40, 1459 RSC.
- S. H. Hwang, C. N. Moorefield, F. R. Fronczek, O. Lukoyanova, L. Echegoyen and G. R. Newkome, Chem. Commun., 2005, 6, 713–715 RSC.
- K. Mahata, M. L. Saha and M. Schmittel, J. Am. Chem. Soc., 2010, 132, 15933–15935 CrossRef CAS PubMed.
- M. Schmittel and K. Mahata, Chem. Commun., 2010, 46, 4163–4165 RSC.
- K. Mahata and M. Schmittel, J. Am. Chem. Soc., 2009, 131, 16544–16554 CrossRef CAS PubMed.
- R. D. Sommer, A. L. Rheingold, A. J. Goshe and B. Bosnich, J. Am. Chem. Soc., 2001, 123, 3940–3952 CrossRef CAS PubMed.
- E. C. Constable, B. A. Hermann, C. E. Housecroft, M. Neuburger, S. Schaffner and L. J. Scherer, New J. Chem., 2005, 29, 1475–1481 RSC.
- J. L. Wang, X. Li, X. Lu, I. F. Hsieh, Y. Cao, C. N. Moorefield, C. Wesdemiotis, S. Z. D. Cheng and G. R. Newkome, J. Am. Chem. Soc., 2011, 133, 11450–11453 CrossRef CAS PubMed; Y. Li, Z. Jiang, J. Yuan, D. Liu, T. Wu, C. N. Moorefield, G. R. Newkome and P. Wang, Chem. Commun., 2015, 51, 5766 RSC.
- S. H. Hwang, P. S. Wang, C. N. Moorefield, L. A. Godinez, J. Manriquez, E. Bustos and G. R. Newkome, Chem. Commun., 2005, 37, 4672–4674 RSC.
- Y. T. Chan, C. N. Moorefield, M. Soler and G. R. Newkome, Chem.–Eur. J., 2010, 16, 1768–1771 CrossRef CAS PubMed.
- Y. T. Chan, X. Li, M. Soler, J. L. Wang, C. Wesdemiotis and G. R. Newkome, J. Am. Chem. Soc., 2009, 131, 16395–16397 CrossRef CAS PubMed.
- G. R. Newkome, T. J. Cho, C. N. Moorefield, G. R. Baker, R. Cush and P. S. Russo, Angew. Chem., Int. Ed., 1999, 38, 3717–3721 CrossRef CAS.
- V. Stepanenko and F. Wurthner, Small, 2008, 4, 2158–2161 CrossRef CAS PubMed.
- S. Perera, X. Li, M. Guo, C. Wesdemiotis, C. N. Moorefield and G. R. Newkome, Chem. Commun., 2011, 47, 4658–4660 RSC; Y. T. Chan, X. Li, C. Wesdemiotis, C. N. Moorefield and G. R. Newkome, Chem.–Eur. J., 2011, 17, 7750–7754 CrossRef CAS PubMed.
- K. Suzuki, M. Tominaga, M. Kawano and M. Fujita, Chem. Commun., 2009, 13, 1638–1640 RSC.
- S. L. Zhang, R. F. Chen, J. Yin, F. Liu, H. J. Jiang, N. E. Shi, Z. F. An, C. Ma, B. Liu and W. Huang, Org. Lett., 2010, 12, 3438–3441 CrossRef CAS PubMed.
- Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520–554 CrossRef CAS PubMed.
- H. Lee, R. M. Venable, A. D. MacKerrell Jr and R. W. Pastor, Biophys. J., 2008, 95, 1590–1599 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of 1H NMR, 2D COSY, MS, isotope pattern and UV data were included here. See DOI: 10.1039/c5ra21307j |
| ‡ These author contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |