In vitro antileishmanial activity of aza-scorpiand macrocycles. Inhibition of the antioxidant enzyme iron superoxide dismutase†
Abstract
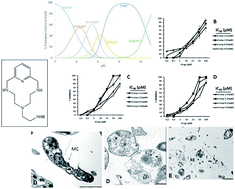
The in vitro leishmanicidal activity of a series of nine aza-scorpiand-like macrocycles, recently synthesized, was tested on Leishmania infantum, Leishmania braziliensis and Leishmania donovani parasites, using promastigotes and intracellular amastigotes forms. The cytotoxicity of the tested compounds on J774.2 macrophage cells was also measured. Four of the tested compounds (1, 2, 8 and 9) showed selectivity indexes higher than those of the reference drug Glucantime for the three Leishmania species. Moreover, the data on infection rates and on amastigotes showed that compounds 1, 2, 8 and 9 are the most active against the three Leishmania species. The changes in the excretion product profile of parasites treated with the four compounds (1, 2, 8 and 9) were also consistent with substantial cytoplasmic alterations. On the other hand, the most active compounds were potent inhibitors of Fe-SOD in the three parasite species considered, whereas their impact on human CuZn-SOD was low. The high activity, low toxicity, stability, low cost of the starting materials and straightforward synthesis make these compounds appropriate molecules for the development of affordable anti-leishmanicidal agents.

 Please wait while we load your content...
Please wait while we load your content...