Assessment of SOC adsorption prediction in activated carbon filtration based on Freundlich coefficients calculated from compound properties
Abstract
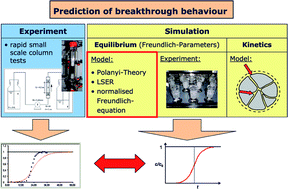
Three models using compound properties to calculate Freundlich constants for adsorption of SOCs on activated carbon were evaluated using data obtained from the literature and in our own experiments. The models are based on Polanyi's potential theory, Linear Solvation Energy Relationships (LSERs) and a solubility normalisation of the Freundlich equilibrium model. Using the extensive collection of data it could clearly be shown which datasets deviate from the general behaviour and that those deviations were due to methodical differences, rather than due to different behaviour of some compounds or random behaviour. However, the theories should not be used to predict Freundlich coefficients of complexing compounds such as EDTA. Model adequacy tests showed that the mass of compound adsorbed per mass of carbon was best described when Freundlich constants obtained from LSERs were used. The comparison of experimental and predicted breakthrough curves using equilibrium Freundlich constants from the three models investigated showed that the prediction of breakthrough behaviour based on Freundlich coefficients predicted from compound properties and the Linear Driving Force (LDF) model for the kinetics is suitable in terms of a conservative risk assessment. A general procedure is proposed to predict breakthrough of compounds for which Freundlich constants are not available.

 Please wait while we load your content...
Please wait while we load your content...