Highly effective utilization of ethylene tar for mesophase development via a molecular fractionation process†
Chuanzhang Gea,
Haixiao Yanga,
Jitong Wanga,
Wenming Qiaoab,
Donghui Long*a and
Licheng Linga
aState Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: longdh@mail.ecust.edu.cn; Fax: +86-21-6425-2914; Tel: +86-21-6425-2924
bShanghai Key Laboratory of Multiphase Materials Chemical Engineering, Shanghai, China
First published on 18th December 2015
Abstract
Ethylene tar (ET) is a bulk chemical by-product from the ethylene industry. Realizing effective utilization of ET will have great significance to both economic and environmental benefits. In this paper, a facile molecular fractionation process is proposed for the effective utilization of ET and for the development of flow-domain textured mesophase pitches. Through vacuum distillation, ET was separated into the light fraction (LF) and the heavy cut (HC), in which the LF rich in alkylated benzenes, naphthalene and methylnaphthalene could be refined to produce useful chemicals, and the HC residuals could serve as good precursors for high-valued mesophase pitches. It was found that the distillation temperature strongly affected the HC's components especially the content of cata-condensed and peri-condensed compounds, resulting in different feasibilities for mesophase development. HC obtained at a distillation temperature of 250 °C demonstrated the best ability for quick formation and coalescence of the mesophase, which thus was chosen as the precursor for mesophase pitch preparation. Furthermore, a series of operating conditions including temperature, duration and pressure (pressurization and depressurization) were systematically optimized. High performance mesophase pitch with high softening point, high carbon yield, low ash content and 100% anisotropy of flow domain texture was successfully produced under ambient conditions at 400 °C for a duration of 12 h.
1. Introduction
Ethylene tar (ET), a typical residual oil from the naphtha cracking process (ca. 15% in yield), is produced on a large scale each year with the growing need for ethylene.1 According to statistical data, the global ET yield is predicted at over 30 million tons per year by 2017.2 The advantage features of this residue, such as being completely soluble in toluene, a high carbon/hydrogen ratio, high aromaticity and essentially free from inorganic ash, make it a suitable source for high-valued carbon materials.3,4 However, up to now ET is mostly used in the manufacture of carbon black or consumed as an industrial combustion fuel. This not only results in low economic value but also contributes to serious environmental problems such as CO2 emissions and global warming. Therefore, from both economic and environmental viewpoints, developing higher value products from ET is urgently required at present.Mesophase pitch, which is composed of planar disc-like aromatic compounds of high molecular weight, is regarded as an excellent precursor for the preparation of advanced functional carbon materials, such as high density and high strength graphite materials,5,6 carbon anodes,7 high thermal conductivity carbon fibers8 and carbon/carbon composites.9,10 However, the high price of the commercialized high performance mesophase pitch has become the primary factor that limiting its wide applications. Therefore, how to prepare low cost high performance mesophase pitch is still a challenge task.
Generally mesophase pitch can be prepared from various organic sources of heavy oil, coal tar and petroleum pitch by thermal carbonization or pure aromatic compounds through catalytic polymerization. The thermal transformation of pitch into mesophase has been well studied since the pioneering investigation of Brooks and Taylor.11 It has been common accepted that the development of mesophase from aromatic feedstock is results of sequential chemical and physical changes during the thermal treatment, including polymerization, nucleation and grow in size by absorbing the isotropic matrix. This process is largely determined by the combined influence of chemical composition of the raw materials and the process conditions.12
However, the utilization of ET as the feedstock for the preparation of high performance mesophase materials is very limited, because of its high carbonization reactivity which playing a negative role in the growth of mesophase. ET is a very complex mixture with wide molecular distribution and abundant reactive side chains, especially including low boiling-point olefin functional groups substituted aromatic components4 and excessive reactive cata-condensed components,12,13 which result in their high and uncontrollable reactivity. In the past three decades, various methods, including hydrogenation treatment,4 co-carbonization with coal tar3,14 and even catalyzation by using AlCl3,15,16 have been applied attempting to modify ET to facilitate the development of large anisotropic texture mesophase. Nevertheless, those investigations result in a high operation cost, high residue of heteroatoms and the difficulty to remove by-products or additives from the products, limiting the wide application of these methods in industrial scale.
According to the utilization of other aromatic feedstock,17–19 the modification of complex precursors by using thermal pre-treatment or cutting components through distillation and extraction has been demonstrated to be effective for the molecular reformation. These approaches would result in materials with proper thermal reactivity, which can be suitable for the preparation of high quality mesophase products. However, only few researches have been done for ET in term of its high value utilization, especially for the development of mesophase pitches.
In this paper, a facile molecular fractionation process is proposed for the effective utilization of ET and for the development of flow-domain textured mesophase pitches. Firstly, ET is separated into light fraction (LF) and residual heavy cut (HC) through a vacuum distillation, particularly focusing on the effect of distillation temperature on the physical–chemical characteristics of the ET derivatives. The LF, which riches in two rings alkylated species, is regarded as valuable source for developing useful chemicals such as alkylated benzenes, naphthalene and methylnaphthalene. While the HC part composed of various heavy molecules with appropriate thermal reactivity, is served as alternative precursor for mesophase development. The link between HCs' composition and the quality of the generated mesophase is investigated. The scaled-up preparation of high performance mesophase pitch is then explicitly studied by using the optimal HC under different heat-treatment conditions. This route should suggest a significant guidance for the comprehensive utilization of ET and potential market can be stimulated by the developing of high value carbon materials.
2. Experimental
2.1. Raw material and pre-treatment
For the purpose of this work, the ET obtained from Sinopec Shanghai Petrochemical Co. Ltd. (PR China) was used as the raw material. The viscous raw ET was first cut into light fraction (LF) and heavy cut (HC) by vacuum distillation under pressure of 0.025 mmHg at an end temperature of 200 °C, 250 °C and 300 °C for 0.5 h, respectively. The LFs and HCs were labelled as LF-200, LF-250, LF-300, HC-200, HC-250 and HC-300, respectively.2.2. Preliminary carbonization
To select a proper precursor for the mesophase pitch preparation, about 5 g of each HC-200, HC-250 and HC-300 were carbonized in a horizontal quartz tube (500 mm long, 30 mm diameter) at 400 °C for 10 h, 410 °C for 5 h and 410 °C for 10 h under a nitrogen flow of 100 mL min−1 at a heating rate of 2 °C min−1, respectively.2.3. Mesophase pitch preparation
The mesophase pitches were prepared through the thermal carbonization of the optimal HC in a stirred tube bomb reactor of 150 mm height and 70 mm diameter. The reactor was heated by an electric furnace. In a typical run, 200 g HC was used. First, the solid HC was grounded to powders of less than 5 mm in diameter and re-melted at 250 °C under agitation of 350 rpm for 1 h with a nitrogen flow of 150 mL min−1. Then the thermal carbonization was carried out at atmospheric pressure by heating the samples to 360 °C, 380 °C and 400 °C respectively at a heating rate of 1 °C min−1 and maintaining the pitch at the end temperature for 12 h under a continuous nitrogen flow. For comparison, pitch for residence time of 6 h was prepared under 400 °C. The pitch samples were labeled as S360-12, S380-12, S400-12 and S400-6 respectively.Also pitches under an autogenic pressure of 2.1 MPa and vacuum condition were prepared at temperature of 380 °C for duration of 12 h to study the effect of pressure on the mesophase development. The pitches were named as S380-12-P and S380-12-V, respectively. The pyrolysis light oil were distilled from the carbonization system and collected in a separated vessel. All the samples except S380-12-V were distilled at 340 °C under vacuum for 30 min to remove volatile fractions at the end of the reaction.
2.4. Characterization
GC/MS spectrums of the raw ET, LFs and HCs were recorded in a Shimadzu GCMS-QP2010 plus instrument, equipped with a DB-5 mass spectrometry column and a split/splitless injector. Standard mass spectra were obtained using 70 eV ionization energy. The inlet temperature was kept at 300 °C with helium used as the carrier gas. Sample of 1 μL was injected into the injector with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. The temperature of the column was initially held at 100 °C for 5 min and then programmed to 260 °C at a rate of 7 °C min−1 and held for 3 min. The mass spectrometer was scanned repetitively from 35 to 800 amu at a rate of 0.30 s per scan velocity. The identification of the peaks was based on computer matching of the mass spectra with the National Institute of Standards and Technology (NIST) database. Quantitative analysis of each essential component in the samples (expressed as area percentage) was obtained from peak area normalization measurement.
30. The temperature of the column was initially held at 100 °C for 5 min and then programmed to 260 °C at a rate of 7 °C min−1 and held for 3 min. The mass spectrometer was scanned repetitively from 35 to 800 amu at a rate of 0.30 s per scan velocity. The identification of the peaks was based on computer matching of the mass spectra with the National Institute of Standards and Technology (NIST) database. Quantitative analysis of each essential component in the samples (expressed as area percentage) was obtained from peak area normalization measurement.
Softening points of the pitches were measured by a thermomechanical analyzer (TMA) (DMA2980, America). The sample was initially softened and solidified in an aluminium crucible, which then was placed in the TMA chamber with a probe on the surface of the pitch to give a constant penetration pressure of 0.001 N. Heating was carried out from ambient to 350 °C at 5 °C min−1 in nitrogen atmosphere. The softening points were finally determined by the intersection of tangents before and after the penetration.
The carbon and hydrogen contents of the prepared pitches were determined using an elemental analyser (elementar vario EL III, Germany). The pitch was fractionated by sequential Soxhlet extraction using toluene, pyridine and quinoline.
Toluene soluble fractions (TS) of the pitches were characterized using 1H NMR on a Bruker Avance 400 spectrometer. The sample was dissolved in CDCl3 and then placed in a 5 mm NMR tube. Chemical shifts were measured using tetramethylsilane (TMS) as an internal standard.
The crystal structures of the prepared pitches were characterized by X-ray diffraction (XRD) analysis using a Bruker D8 Advance diffractometer with Cu Kα radiation (λ = 1.54056 Å). The data were collected between scattering angles (2θ) of 5 to 80°. To determine the crystallite parameters, the interlayer distance (d002) between planar stacked layers was calculated by Bragg equation (eqn (1)),20 in which λ is the wavelength of X-ray and θ is the Bragg angle of 002 band around 26° in the 2θ axis. The average height of the stacked planar layers (Lc) perpendicular to the layer plane was calculated by using Scherrer's equation (eqn (2)),21 where β is full width at half maximum (FWHM) of 002 band.
d002 = λ/(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
Lc = 0.89λ/(β002![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (2) |
The number of aromatic sheets in the stacked cluster, N, was calculated by the following equation:
| N = (Lc/d002) + 1 | (3) |
The optical microstructures of the mesophase samples were analyzed by using Olympus BX51 microscope which equipped with a polarizer and one-way retarder plate. The samples were mounted in epoxy resin and smoothed using progressively finer grades of silicon carbide paper, and then polished using alumina powder on a cloth pad. Representative images of the samples were taken using objective of 20× magnification. The mesophase content was determined from the analysis of 500 points.22
3. Results and discussion
3.1. Composition of original ET and the distillation cuts
Generally, the composition of ET is closely depended on the applied reactor type, feeding materials and the processing conditions. Thus the typical ET studied in this work was sampled from the stable large-scaled production unit (Sinopec Shanghai Petrochemical Co. Ltd., PR China). The main components of the original ET were identified by GC/MS, as shown in Fig. 1(a). A list of the identified PAHs is given in Table S1.† The integration results based on the area of the whole spectrum indicate that ET is mostly composed of 2–3 rings multimethyl-substituted polyaromatic hydrocarbons (PAHs), including naphthalene, methylnaphthalene and anthracene. A number of weak peaks are also found at retention time less than 4 min and larger than 21 min, which could be assigned to olefins such as 2-methylstyrene, indene and (1-methyl-1,2-propadienyl)benzene, and heavy PAHs of more than 3-rings, including pyrene, methylpyrene, benzanthracene, and methylchrysene, respectively. These mixtures with different molecular structures will give rise to a very complicated thermal reaction behaviour of ET. | ||
| Fig. 1 (a) GC/MS spectrum of ET, (b) proposed route for effective utilization of ET and (c) distillation yield of light oil under different temperatures. | ||
As shown in Fig. 1(b), a simple molecular fractionation process through vacuum distillation is proposed to realize highly effective usage of ET. The light oils, which consisted of small molecules, can be easily distilled out at 200–300 °C under vacuum (0.025 mmHg). It should be noted that the distillation temperature is a very important factor to determine the yield and the composition of light oils. As indicated by Fig. 1(c), about 37 wt% of ET could be distilled at 200 °C, while it increases to 64 wt% at 300 °C. The molecular compositions of these light fractions (LFs) and the resulting residual heavy cuts (HCs) are analyzed by GC/MS as shown in Fig. 2(a). The identified PAHs in the LFs and HCs are also given in Table S1.† Apparently, olefins and 2-rings PAHs including naphthalene, 1-methylnaphthalene, dimethylnaphthalene could be easily distilled out. Some 3-rings cata-condensed PAHs such as anthracene could be also removed when the distillation temperature is up to 250 °C. These low-boiling-point LFs could be further separated by vacuum or extractive distillation to obtain high-purity chemical materials such as naphthalene, α-methylnaphthalene and β-methylnaphthalene, which could greatly improve the economic utilization of ET. Similar works have been well-reported before23–26 thus the further purification of the light fraction is not the focus of the present work.
The residual HCs are dark brown solids with 100% soluble in toluene. As analyzed by GC/MS in Fig. 2(b), these HCs are majorly consisted of 3–5 rings PAHs. The possible compositions of these HCs are grouped in Table 1. As for HC-200, the main components are 54.9% of 3-rings and 41.0% of 4-rings. While for HC-250, the 3-rings species decrease significantly to 23.9% and the 4-rings species become the dominated components with the fraction up to 67.0%. As the distillation temperature increases to 300 °C, the content of 4-rings species increases to 72.0% and only 2.0% of 3-rings species remain. Thus, the molecular compositions of HC are strongly depended on the distillation temperature. Table 1 also lists some physical properties of these HCs. The softening points of these solid residual HCs are measured to be 65 °C, 101 °C and 135 °C for HC-200, HC-250 and HC-300, respectively, which increase proportionally with the distillation temperature. The improved softening point, high C/H ratio (shown in Table S2†) and low ash content suggest these materials might be an excellent precursor for fabricating advanced carbon artifacts. However, the direct carbonization yields of these HCs (measured by TG at 800 °C in N2 flow, Fig. S2†) are low of only 13–25% which make them unsuitable to be used directly for this application. Thus, the thermal conversion of these materials to high-softening-point mesophase pitch is an alternative way to improve the added value of these heavy residues.
| Samples | Area percentage of the componenta | SPb (°C) | TSc (wt%) | CYd (wt%) | Ash (wt%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |||||
| a R1 to R5 refer to one to five rings species, respectively.b Softening point determined by hot stage.c Toluene soluble fraction.d Carbon yield at 800 °C. | |||||||||
| ET | 5.9 | 68.8 | 16.5 | 7.8 | 1 | <10 | 100 | 5.5 | 0.01 |
| LF-200 | 7.8 | 88.2 | 4 | 0 | 0 | — | 100 | — | — |
| LF-250 | 6.4 | 79.0 | 14.5 | 0 | 0 | — | 100 | — | — |
| LF-300 | 6.5 | 69.6 | 18.4 | 5.5 | 0 | — | 100 | — | — |
| HC-200 | 0 | 0 | 54.9 | 41 | 4.1 | 65 | 100 | 13 | 0.02 |
| HC-250 | 0 | 0 | 23.9 | 67 | 9.1 | 101 | 100 | 20 | 0.02 |
| HC-300 | 0 | 0 | 2 | 72 | 26 | 135 | 100 | 25 | 0.03 |
The thermal reactivity, which is determined by the molecular structure, is regarded as a very important factor for the further mesophase transformation process.13,27 According to the identified PAHs shown in Table S1,† the compounds in the HCs can be separated into two groups of cata- and peri-condensed compounds depending on the molecular structure.18 More specifically, the cata- and peri-condensed compounds can be further assigned into four categories, namely cata1: made up of cata-condensed compounds with naphthenic groups and/or substituted with alkyl and aryl groups; cata2: constituted by unsubstituted and planar cata-condensed compounds; peri1: made up of peri-condensed compounds substituted with alkyl and aryl groups; and peri2: constituted by unsubstituted and planar peri-condensed compounds, respectively. Yokono et al.13 previously studied the thermal reactivity of PAHs in terms of the calculated free valence. They suggested that values of maximum free valence as the active sites in the cata-type PAHs (such as anthracene and pentacene) are larger than those of the peri-type compounds (such as pyrene and perylene). In particular, the presence of alkyl side chains is also considered to lead to an increased thermal reactivity.28 Thus there might be an optimal relationship between the structural distribution of the precursor materials and the optical properties of the resultant products. Here, the quantitative analysis of these classifications allows the structural composition of the HCs to be estimated. Fig. 3 shows the relative contents of cata- and peri-condensed compounds. It is found that the cata-condensed compounds are obviously decreased with the increasing temperature of the vacuum distillation. The ratio of total cata-condensed components to total peri-condensed components is calculated of 2.28, 1.32 and 0.98 for HC-200, HC-250 and HC-300, respectively, which suggest a decrease in reactivity for the HCs with higher distillation temperature.
 | ||
| Fig. 3 Distribution of cata- and peri-condensed compounds and ratio of total cata- to peri- of the derived HCs. | ||
3.2. Mesophase development from the residual HCs
To evaluate the feasibility of mesophase generation, the carbonization behaviour of these HCs was investigated by using a horizontal quartz tube in the 400–410 °C range for 5–10 h. The resultant products are observed by optical microscope, which provides information about mesophase growth and its structure, as shown in Fig. 4. Mesophase development during thermal treatment is rather different for the samples studied. The micrographs show a large number of tiny discrete mesophase spheres (less than 40 μm in diameter) throughout the isotropic phase for the product derived from HC-200 under temperatures of 400 °C for 5 h, which should be corresponding to an early growth stage of the mesophase. The elevated temperature enables the spheres growing to larger sizes with maximum about 200 μm under 410 °C. However, no signal of coalescence between the isolated smooth spheres is observed. As the duration is doubled, small separate irregular domains within the isotropic matrix are formed. While for precursor HC-300, obvious coalescing processes are observed (indicated by the white arrows) in the products. The mesophase spheres have grown to sizes ranging from 50 to 300 μm under the same carbonization conditions. Nevertheless, the observation of large amount of isolated spheres together with the nucleating of many new spheres (below 5 μm) also indicates a low degree of mesophase development for HC-300. On the contrast, extra-large spheres of more than 450 μm are formed after the carbonization of HC-250 under 400 °C for 5 h. To be noted, significant non-circular contour of the sections are found in the spheres due to multiple-coalescences, which would undergo a breaking-up process and completely transform into large flow domains under 410 °C for 10 h. These results suggest the mesophase formation ability of the three precursors follows the order HC-250 > HC-300 > HC-200.Although carbonization temperature and soaking time make some differences to the evolution of mesophase, the intrinsic properties (i.e. composition and molecular structure) which determine the thermal reactivity of the precursors, should play a more critical role on the carbonization behaviours for the HCs. As HC-200 possesses the highest content of cata compounds, the corresponding high intermolecular reactivity would give rise to polycondensed aromatic macromolecules (mesogens)18 and thus result in a quick nucleation process (indicated by the large amount of mesophase microspheres in Fig. 4). However, the high reactivity might also result in extensive cross-linking of intra- and intermolecules thus leading to a rapid increase of matrical viscosity, which would hinder the normal growth of the small mesophae spheres.12 In contrast, HC-300, which has the lowest content of cata-condensed compounds, is suggested to exhibit the lowest thermal reactivity. Although the mesophase has grown into numbers of large spheres in diameter of maximum about 300 μm, the insufficient reactivity reduces the nucleation rate and thus delays the further growth of mesophase. On the other hand, it is because of the low thermal reactivity, the mesophase spheres thus have sufficient mobility to coalesce with each other and result in the formation of larger spheres. While for HC-250, the content of cata-type compounds is slight larger than that of peri-type compounds in a ratio of total cata/peri approximate to 1.3. According to the well-developed mesophase from HC-250 under the same conditions, it seems that such composition distribution should play a role in easing the carbonization and give rise to the formation of mesophase in a high yield and with morphology of large flow domain texture, demonstrating a proper thermal reactivity of this precursor. Therefore, HC-250 is used as the suitable precursor for the enlarged preparation of mesophase pitches in the following study.
3.3. Mesophase pitch prepared from HC-250
The scaled-up preparation of mesophase pitch is carried out in a tube bomb reactor by using 200 g HC-250 per batch, which is thermally polymerized to mesophase pitch by ways of nitrogen blowing, pressurization and vacuum treatment, respectively. According to the viscosity versus temperature curve of HC-250 shown in Fig. S3,† the temperatures applied for the scaled-up preparation are proposed in the range of 360–400 °C. The detailed heat-treatment conditions are listed in Table 2.| Samples | Carbonization conditionsa | Yield (wt%) | ||||
|---|---|---|---|---|---|---|
| T | t | P | Pitch | Oil | Gasb | |
| a T: temperature; t: soaking time; P: pressure.b By difference. | ||||||
| S360-12 | 360 °C | 12 h | Atmospheric | 57 | 33 | 10 |
| S380-12 | 380 °C | 12 h | Atmospheric | 55 | 34 | 11 |
| S380-12-P | 380 °C | 12 h | 2.1 MPa | 59 | 32 | 9 |
| S380-12-V | 380 °C | 12 h | Vacuum | 36 | 50 | 14 |
| S400-6 | 400 °C | 6 h | Atmospheric | 56 | 35 | 9 |
| S400-12 | 400 °C | 12 h | Atmospheric | 54 | 36 | 10 |
Fig. 5 shows the optical microstructures of the resultant pitches prepared by different conditions. It can be seen that the development of mesophase varies greatly with the reaction temperatures. At a relative low temperature of 360 °C, the as-prepared pitch does not show any trace of mesophase (Fig. 5(a)). However, when increased the temperature to 380 °C, optical texture of large region coalesced mesophase (80 vol% mesophase) is observed (Fig. 5(b)). To be noted, the mesophase shows signs of mechanical elongation and distortion as a result of agitation. With the temperature further increased to 400 °C, the mesophase is developed into almost flow domain-like texture (Fig. 5(f)), indicating full growth of mesogens and complete coalescence of the mesophase spheres.
In addition, the duration also plays a vital role on the mesophase development. Fig. 5(e) and (f) clearly show the different anisotropic morphologies obtained from duration of 6 h and 12 h at 400 °C, respectively. For a short duration of 6 h, it is observed that the mesophase are mainly in a growing stage with many new formed mesophase spheres coalescing along the edges of the mesophase domain, and considerable amount of the pitch is still remained in an isotropic phase (∼72 vol% mesophase). Only a long duration (12 h) could guarantee the full growth and coalescence of mesophase spheres to form a flow-domain mesophase texture.
The pressure factor is also important to influence the formation of mesophase texture. Fig. 5(c) and (d) compare the optical results of the pitches prepared under pressurized and vacuumed conditions, respectively, both of them cannot form a flow-domain mesophase texture. Under the pressurized condition, most of the pitch is in an isotropic phase, and only a small portion of mesophase microspheres (∼7 vol%) can be observed. This should be due to the extensive incorporation of low-molecular-weight components into pitch framework, which results in a high degree dispersion of the large planar aromatic molecules, and thus prohibits the nucleation of mesophase microspheres. On the other hand, the depressurized condition leads to the formation of coarse mosaic texture with 89 vol% mesophase. The rapid removing of volatile products from the isotropic matrix could promote the nucleation of mesogens. However, the loss of volatile molecules also simultaneously increases the viscosity of the isotropic phase, thus hindering the coalescence of mesophase spheres.30,31
Table 3 summarizes the general physical properties of the prepared pitches. As expected, the softening points, ranging from 161 °C to 299 °C, are increased substantially with the increasing severity of the heat treatment. The carbon yields of the pitches, which varied from 48.6% to 75.4%, are found to consistent with the change of mesophase content. The results of elemental analysis show a high content of total carbon and hydrogen, which is regarded to benefit from the low ash content of the parent material. The C/H atomic ratios gradually increase from 1.61 to 2.08 with the increasing of heat-treatment temperature, indicating a deeper dehydrogenation during the higher temperature polymerization process. The group components of the as-prepared pitches are investigated by using standard Soxhlet extraction method. According to the results, the QI contents generally increases with the increasing of mesophase content. The highest QI content of 55.8 wt% is found in the S400-12, indicating that most of the light molecules are condensed to insoluble ones during the heat treatment.32
| Samples | MCa/vol% | SPb/°C | CYc/wt% | Elemental analysis | Ash/wt% | Solubilityd/wt% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C/wt% | H/wt% | C/H | TS | TI-PS | PI-QS | QI | |||||
| a Mesophase content.b Softening point determined by TMA.c Carbon yield at 800 °C.d TS: toluene soluble fraction; TI-PS: toluene insoluble-pyridine soluble fraction; PI-QS: pyridine insoluble-quinoline soluble fraction; QI: quinoline insoluble fraction. | |||||||||||
| S360-12 | 0 | 161 | 48.6 | 94.77 | 4.92 | 1.61 | 0.04 | 67.7 | 8.0 | 9.4 | 14.9 |
| S380-12 | 80 | 272 | 61.9 | 95.42 | 4.18 | 1.90 | 0.04 | 45.2 | 8.0 | 15.5 | 31.3 |
| S380-12-P | 7 | 186 | 53.0 | 95.12 | 4.53 | 1.75 | 0.05 | 59.3 | 12.4 | 14.7 | 13.6 |
| S380-12-V | 89 | 288 | 71.5 | 95.62 | 4.06 | 1.96 | 0.06 | 32.2 | 4.9 | 14.4 | 48.5 |
| S400-6 | 72 | 253 | 56.2 | 95.28 | 4.36 | 1.82 | 0.04 | 55.5 | 10.6 | 13.3 | 20.6 |
| S400-12 | 100 | 299 | 75.4 | 95.82 | 3.84 | 2.08 | 0.05 | 28.6 | 3.5 | 12.1 | 55.8 |
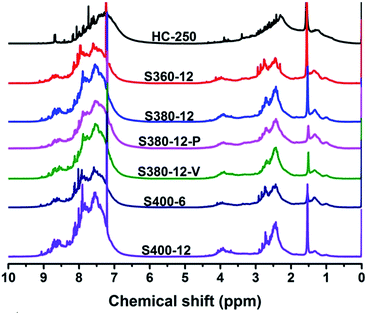
| Samples | 1H NMR | XRD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Har | Hα | Hβ | Hγ | HCH2 | Har/Hal | d002 (nm) | Lc (nm) | N | |
| HC-250 | 0.442 | 0.341 | 0.186 | 0.031 | 0.031 | 0.79 | — | — | — |
| S360-12 | 0.585 | 0.292 | 0.102 | 0.021 | 0.045 | 1.41 | 0.3610 | 0.74 | 3 |
| S380-12 | 0.650 | 0.272 | 0.067 | 0.012 | 0.044 | 1.85 | 0.3508 | 1.27 | 5 |
| S380-12-P | 0.608 | 0.278 | 0.094 | 0.020 | 0.040 | 1.55 | 0.3515 | 1.06 | 4 |
| S380-12-V | 0.678 | 0.256 | 0.056 | 0.010 | 0.043 | 2.11 | 0.3504 | 1.59 | 6 |
| S400-6 | 0.640 | 0.270 | 0.075 | 0.015 | 0.040 | 1.78 | 0.3528 | 1.14 | 4 |
| S400-12 | 0.735 | 0.227 | 0.033 | 0.005 | 0.045 | 2.77 | 0.3469 | 1.72 | 6 |
The ordering of the anisotropic structures in the prepared pitches was investigated by XRD technique, as shown in Fig. 7. For comparison, the XRD pattern of the HC-250 is also present. It is observed that HC-250 exhibits a broad typical γ-band (corresponding to the stack of aliphatic layers35) at ca. 18°, which is transformed to a graphene (002) band at ca. 25° after the heat treatment.36 The intensity of 002 bands in the resultant pitches are sharped instantaneously with the increasing severity of the heat treatment and the employing of depressurization condition, which indicating an increased average crystallite size of the pitches. Based on 002 peaks, the crystal parameters of interlayer spacing (d002), crystallite size (Lc) and average number of aromatic sheets per stacked cluster (N) are calculated and listed in Table 4. It is found that the Lc and N value increase with the increasing of mesophase content due to the greater ordering of the stacked structures.36 The conditions of higher temperature, longer duration and depressurization facilitate the removal of disordered low molecular weight components and thus give rise to more ordered anisotropic products.
4. Conclusions
In a conclusion, we have developed a facile molecular fractionation process for the effective utilization of ethylene tar by separating it into light fractions and heavy cuts through vacuum distillation. The distillation temperature is found to be crucial factor for determining the composition of the derivatives. The LFs, which rich in two rings homologous naphthalene, are suggested to be refined for important chemicals. The HCs, which are composed of heavy PAHs, show a great versatility for generating mesophase with different optical textures. The molecular distribution of the HCs, which determines the thermal reactivity, is found to play a critical role on the mesophase formation. HC-250, which mainly contain 3–5 rings PAHs and a suitable proportion of cata-condensed compounds with respect to peri-condensed compounds produce the highest degree of mesophase development. High performance mesophase pitches with high softening point, high carbon yield and 100% anisotropy of flow domain texture are successfully produced upon a simple heat polymerization. This indicates that the vacuum distillation process is a simple and potentially economical technology to realize high effective utilization of ET and cost-effective production of high performance mesophase materials.Acknowledgements
The finance and encouragement of this work is supported by National Natural Science Foundation of China (U1303291 and No. 51272077).References
- http://www.s00.static-shell.com/content/dam/shell/static/chemicals/downloads/responsible-energy/ethylene-crackerresidueproductstewardshipsummaryapril2012.pdf.
- http://www.energy.globaldata.com/media-center/press-releases/oil-and-gas/us-and-china-driving-global-ethylene-capacity-to-record-208-million-tons-per-year-by-2017-says-globaldata.
- X. Cheng, Q. Zha, J. Zhong and X. Yang, Fuel, 2009, 88, 2188–2192 CrossRef CAS.
- I. Mochida, Q. F. You and Y. Korai, Fuel, 1990, 69, 667–671 CrossRef CAS.
- I. Mochida, R. Fujiura, T. Kojima, H. Sakamoto and K. Kanno, Carbon, 1994, 32, 961–969 CrossRef CAS.
- I. Mochida, R. Fujiura, T. Kojima, H. Sakamoto and T. Yoshimura, Carbon, 1995, 33, 265–274 CrossRef CAS.
- I. T. Dimov, V. N. Alexandrov, I. Mochida, C. Ku, S. Yoon and Y. Korai, J. Power Sources, 1998, 75, 214–222 CrossRef.
- D. D. Edie, K. E. Robinson, O. Fleurot, S. P. Jones and C. C. Fain, Carbon, 1994, 32, 1045–1054 CrossRef CAS.
- W. Q. Li, H. B. Zhang, X. Xiong and F. Xiao, Mater. Sci. Eng., 2011, 528, 2999–3002 CrossRef.
- R. Fujiura, T. Kojima, K. Kanno, I. Mochida and Y. Korai, Carbon, 1993, 31, 97–102 CrossRef CAS.
- J. D. Brooks and G. H. Taylor, Carbon, 1965, 3, 185–193 CrossRef CAS.
- R. García, J. L. Crespo, S. C. Martin, C. E. Snape and S. R. Moinelo, Energy Fuels, 2003, 17, 291–301 CrossRef.
- T. Yokono, K. Miyazawa, Y. Sanada and H. Marsh, Fuel, 1979, 58, 692–694 CrossRef CAS.
- I. Mochida, Q. F. You, Y. Korai and T. Oishi, Fuel, 1990, 69, 672–677 CrossRef CAS.
- I. Mochida, Y. Sone and Y. Korai, J. Mater. Sci. Lett., 1985, 4, 1237–1240 CrossRef CAS.
- I. Mochida, Y. Sone and Y. Korai, Carbon, 1985, 23, 175–178 CrossRef CAS.
- P. Álvarez, J. Sutil, R. Santamaria, C. Blanco, R. Menéndez and M. Granda, Energy Fuels, 2008, 22, 4146–4150 CrossRef.
- M. Pérez, M. Granda, R. Garcıa, R. Santamaría, E. Romero and R. Menéndez, J. Anal. Appl. Pyrolysis, 2002, 63, 223–239 CrossRef.
- V. A. Weinberg, J. L. White and T. F. Yen, Fuel, 1983, 62, 1503–1509 CrossRef CAS.
- M. N. Siddiqui, M. F. Ali and J. Shirokoff, Fuel, 2002, 81, 51–58 CrossRef CAS.
- F. R. Feret, Analyst, 1998, 123, 595–600 RSC.
- C. Blanco, O. Fleurot, R. Menéndez, R. Santamaría, J. Bermejo and D. Edie, Carbon, 1999, 37, 1059–1064 CrossRef CAS.
- J. E. C. Makin, U.S. Pat, 3,005,032, 1961.
- B. S. Friedman, U.S. Pat, 2,920,115, 1960.
- R. A. Ewing, R. D. Morin and G. C. Templeman, U.S. Pat, 2,943,122, 1960.
- J. M. Chambers and J. H. D. Robinson, U.S. Pat, 3,075,890, 1963.
- R. A. Greinke, Carbon, 1986, 24, 677–686 CrossRef CAS.
- W. C. Herndon, Tetrahedron, 1982, 38, 1389–1396 CrossRef CAS.
- K. J. Hüttinger and U. Rosenblatt, Carbon, 1977, 15, 69–74 CrossRef.
- S. R. Bagheri, M. R. Gray and W. C. McCaffrey, Energy Fuels, 2011, 25, 5541–5548 CrossRef CAS.
- R. Santamaría-Ramírez, E. Romero-Palazón, C. Gómez-de-Salazar, F. Rodríguez-Reinoso, S. Martínez-Saez, M. Martínez-Escandell and H. Marsh, Carbon, 1999, 37, 445–455 CrossRef.
- C. Panaitescu and G. Predeanu, Int. J. Coal Geol., 2007, 71, 448–454 CrossRef CAS.
- C. E. Snape, Fuel, 1983, 62, 621–624 CrossRef CAS.
- P. Torregrosa-Rodríguez, M. Martínez-Escandell, F. Rodríguez-Reinoso, H. Marsh, C. G. de Salazar and E. R. Palazón, Carbon, 2000, 38, 535–546 CrossRef.
- F. S. Alhumaidan, A. Hauser, M. S. Rana, H. M. S. Lababidi and M. Behbehani, Fuel, 2015, 150, 558–564 CrossRef CAS.
- A. G. Alvarez, M. Martínez-Escandell, M. Molina-Sabio and F. Rodríguez-Reinoso, Carbon, 1999, 37, 1627–1632 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra20651k |
| This journal is © The Royal Society of Chemistry 2016 |