Preparation of Y-doped ZrO2 coatings on MnO2 electrodes and their effect on electrochemical performance for MnO2 electrochemical supercapacitors
Yuqing Zhang*ab and
Yufeng Zhaiab
aSchool of Chemical Engineering and Technology, Tianjin University, 300072, Tianjin, P. R. China
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, P. R. China. E-mail: zhangyuqing@tju.edu.cn; Fax: +86-22-27403389; Tel: +86-22-27890470
First published on 16th December 2015
Abstract
To enhance the cycling stability and conductivity of manganese dioxide (MnO2) electrodes for supercapacitors, yttrium (Y) doped zirconia (ZrO2) (denoted as Y/ZrO2) is coated on MnO2 supercapacitor electrodes (Y/ZrO2@MnO2 electrodes), so protecting the MnO2 electrodes in the electrolytes and enhancing the electrochemical performance of MnO2 electrodes in sodium sulfate electrolytes. The Y/ZrO2@MnO2 electrodes are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX) and XRD analysis. The electrochemical properties of the electrodes are tested and analyzed by galvanostatic charge/discharge tests, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The Y/ZrO2@MnO2 electrodes achieve a specific capacity of 282.1 F g−1 with a specific capacity loss of only 6.3% after 100 cycles at the current density of 50 mA g−1. The results show that MnO2 particles are successfully deposited by Y-doped ZrO2 while the Y/ZrO2@MnO2 electrodes display better cycling stability and capacity performance. Therefore, this Y-doped ZrO2 coating is a potential choice to improve the cycling stability and conductivity of MnO2 electrodes.
1. Introduction
Since entering the 21st century, with the increasing consumption of fossil fuels for the development of the economy, environment pollution and the energy crisis have become the most important problems that we must deal with. Therefore, there is a great need to look for renewable and sustainable energy sources and develop technologically advanced devices for energy conversion and storage.1–4 In recent years, electrochemical supercapacitors (ESs), as a new sort of efficient device for energy conversion and storage, have caused extensive concern because of their high power density, fast charge and discharge rate, better cycling stability, and longer life-cycle.1,2 These properties and characteristics make ESs applicable to many domains, such as electric vehicles, computers, electrochemical power sources, military applications, power distribution systems, etc.5–7 Therefore, supercapacitors are expected to become a novel energy resource to solve the problems concerning energy storage and environmental pollution.The electrochemical properties of supercapacitors, such as specific capacity and cycling stability, intimately depend on the electrode materials.2 Thus, improving the electrochemical performance of electrode materials is an effective strategy to enhance the electrochemical properties of supercapacitors. Generally, various materials for electrodes of supercapacitors such as carbonaceous materials, conducting polymers and transition-metal oxides have been researched. Among these materials, MnO2, as a sort of transition-metal oxides, is considered as a promising alternative class of materials for electrochemical supercapacitors, for its superior characteristics such as large theoretical specific capacity (1370 F g−1), environmental safety, low toxicity and natural abundance, as well as low cost.8–10 In spite of all of the superiorities, commercial applications of supercapacitors based on MnO2 electrode have not been implemented mostly due to some existing drawbacks about MnO2 as follows. Firstly, partial dissolution of MnO2 electrode in the electrolyte happens spontaneously during cycling, leading to the fading capacity.2,11 Secondly, poor electronic conductivity and ionic conductivity result in large resistance of MnO2 electrode in supercapacitors.12 Hence, it is a key task for researchers to solve these disadvantages of MnO2 material mentioned above.
Among these drawbacks of MnO2 electrode material mentioned above, the partial dissolution in the electrolyte is the most prominent issue and draws great attention of researchers. Pang and Anderson13,14 utilized sol–gel method to prepare thin-film MnO2 electrode, the result of test indicating that more than 10% capacity was lost after 1500 cycles, in 0.1 mol L−1 Na2SO4 electrolyte, at a scanning rate of 50 mV s−1. Reddy et al.15 prepared MnO2 electrode, at a sweep rate of 5 mV s−1, suffering more than 50% capacity loss after 800 cycles. Recently, Hsieh et al.11 also reported the capacity fading of electrochemical capacitors based on the MnO2·nH2O electrodes, ranging from 5% to 30% in 1000 cycles, which was close to current rate and binder content. Moreover, it has been reported that the protective coatings on the electrodes surface are an effective strategy to improve the cycling stability of the electrode and enhance its electrochemical properties.16 Walz et al.17 deposited nanoporous silica coating on solid barium ferrate particles and obviously enhanced its stability, comparing to the uncoated materials. Licht et al. have used ceramic material of zirconia (ZrO2) as protective coatings on the K2FeO4 electrodes, and their investigation results indicate that the ZrO2 coating effectively enhances the stability of these electrodes, simultaneously improves the energy storage capacity of super-iron batteries.18,19 Nevertheless, as we all know, ZrO2 can form cubic, tetragonal, and monoclinic phases or orthorhombic phases. Among these phases, the cubic form has caused the extensive concern attributed to its high conductivity, excellent thermal stability, mechanical properties, chemical resistance, and oxygen conductivity. In spite of all of the superiorities, the stability of the cubic form at room temperature is not satisfactory. Therefore, the stabilization of cubic form at room temperature is very necessary.20,21 It is reported that doping Y to ZrO2 can stabilize cubic of ZrO2 at room temperature; meanwhile the conductivity of cubic ZrO2 at room temperature can be improved as well.22 In addition, Zhang et al.23,24 successfully investigated and developed Y-doped zirconia coatings on K2FeO4 particles, significantly enhancing the stability and conductivity of the coated K2FeO4 electrodes and provided a good strategy for our further research.
Based on the previous work and investigation above, it is necessary to research and develop a novel sort of electrode materials for supercapacitors. Therefore, in order to enhance the cycling stability and conductivity of manganese dioxide (MnO2) electrodes for supercapacitors in this paper, yttrium (Y) doped zirconia (ZrO2) (denoted as Y/ZrO2) are coated on MnO2 electrodes as supercapacitors electrodes (Y/ZrO2@MnO2 electrodes), so protecting the MnO2 electrodes and enhancing the electrochemical performance of MnO2 electrodes in sodium sulfate electrolytes. And then their structure and electrochemical performance were observed and researched through SEM, TEM, EDX, galvanostatic charge/discharge test, CV and EIS.
2. Experimental
2.1 Materials
Zirconium oxychloride octahydrate (ZrOCl2·8H2O) (AR grade, ≥99.0%), ammonia water (NH3·H2O) (AR grade, 25–28%), and cetyltrimethyl ammonium bromide (CTAB) (AR grade, 99.0%) were purchased from Tianjin GuangFu fine chemical research institute. Manganous sulfate (MnSO4·H2O) (AR grade, ≥99.0%), ethanol absolute (C2H5OH) (AR grade, ≥99.7%), and sodium sulfate (Na2SO4) (AR grade, ≥99.0%) were supplied by Tianjin Guangfu Fine Chemicals Co., Ltd. Potassium permanganate (KMnO4) (AR grade, ≥99.0%) and yttrium nitrate (Y(NO3)3·6H2O) (AR grade, ≥99.0%) were obtained from Tianjin Jiangtian chemical Co., Ltd. Acetylene black (AR grade, ≥99.0%, Tianjin Jinqiushi Chemical Co., Ltd.), poly vinylidene difluoride (PVdF) (AR grade, ≥99.0%, Chengdu Chenguang Research Institute of Chemical Industry), nickel foam, and N-methyl pyrrolidone (NMP) (AR grade, ≥99.0%, Tianjin Bo di Chemical Co., Ltd.) were obtained and used as-received.2.2 Preparation of Y/ZrO2@MnO2 electrodes
2.3 Fabrication of electrodes
The electrodes for evaluating the electrochemical properties were fabricated by mixing Y/ZrO2@MnO2 (80 wt%) sample with acetylene black (10 wt%) and PVdF (10 wt%) in a beaker. Then moderate amount of NMP was added into the electrode material to form slurry. After that, the slurry was coated (coating area: 1 × 1 cm2) on a nickel foam (1.8 mm, washed by ethyl alcohol under sonication for 1 h). Next, the electrodes were dried at 60 °C for 3 h under vacuum (vacuum degree: ≥0.08 MPa), and then pressed at 10 MPa for 10 seconds. Finally, the electrodes were dried at 60 °C for 12 h in the air.2.4 Characterization
2.5 Electrochemical tests
Electrochemical tests of the as-prepared electrodes, including the galvanostatic charge/discharge test, the cyclic voltammetry (CV) test, and the electrochemical impedance spectroscopes (EIS) test. All the electrochemical tests were performed using 0.5 mol L−1 aqueous Na2SO4 solution as the electrolyte at 25 °C in this study.3. Results and discussion
3.1 Determination of preparation conditions of Y/ZrO2@MnO2 particles
The electrochemical performances of Y/ZrO2@MnO2 electrodes are affected by many factors during depositing Y/ZrO2 coatings on the surface of MnO2 electrodes. Among those factors, the adding amount of ZrOCl2·8H2O, the doping amount of Y(NO3)3·6H2O (the mole ratio of Y(NO3)3·6H2O and ZrOCl2·8H2O) (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol), the concentration of CTAB, the reaction time, and the calcination temperature were observed and researched. To determine the fitting preparation conditions of Y/ZrO2@MnO2 particles, a single factor experiment was used for research and five different levels of every factor were chosen in experiments. Finally, electrodes were prepared using Y/ZrO2@MnO2 particles to further investigate.
mol), the concentration of CTAB, the reaction time, and the calcination temperature were observed and researched. To determine the fitting preparation conditions of Y/ZrO2@MnO2 particles, a single factor experiment was used for research and five different levels of every factor were chosen in experiments. Finally, electrodes were prepared using Y/ZrO2@MnO2 particles to further investigate.
In all the single factor experiments, the specific capacity of produced electrodes mentioned above were evaluated by using the method of galvanostatic charge/discharge test.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) is 3
mol) is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. Then the specific capacity of the electrodes starts to decrease with the doping amount of yttrium being increased unceasingly. Especially, the mole ratio between Y(NO3)3·6H2O and ZrOCl2·8H2O (mol
100. Then the specific capacity of the electrodes starts to decrease with the doping amount of yttrium being increased unceasingly. Especially, the mole ratio between Y(NO3)3·6H2O and ZrOCl2·8H2O (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) is more than 5
mol) is more than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the specific capacity of the electrodes decreases sharply. The reason suggests that: the oxygen vacancies are few in the coating when the doping amount of yttrium (Y) is less, so the resistance of charge transfer is strong, which makes the specific capacity low. With increasing the doping amount of Y(NO3)3·6H2O, the coatings of the electrodes contain more yttrium (Y), which increases the oxygen vacancies and decreases the resistance of charge transfer. But when the doping amount of Y(NO3)3·6H2O is too much, the coatings turn into mixtures of zirconium (Zr) and yttrium (Y), which makes the conductivity in the coatings of the electrodes decrease seriously. As the result, the resistance of charge transfer grows highly and the specific capacity decreases much. Thereby, the fitting mole ratio between Y(NO3)3·6H2O and ZrOCl2·8H2O (mol
100, the specific capacity of the electrodes decreases sharply. The reason suggests that: the oxygen vacancies are few in the coating when the doping amount of yttrium (Y) is less, so the resistance of charge transfer is strong, which makes the specific capacity low. With increasing the doping amount of Y(NO3)3·6H2O, the coatings of the electrodes contain more yttrium (Y), which increases the oxygen vacancies and decreases the resistance of charge transfer. But when the doping amount of Y(NO3)3·6H2O is too much, the coatings turn into mixtures of zirconium (Zr) and yttrium (Y), which makes the conductivity in the coatings of the electrodes decrease seriously. As the result, the resistance of charge transfer grows highly and the specific capacity decreases much. Thereby, the fitting mole ratio between Y(NO3)3·6H2O and ZrOCl2·8H2O (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) is 3
mol) is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
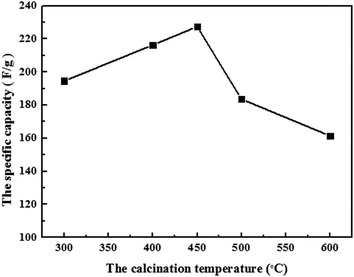
Actually, different crystal structures of MnO2 are formed under different calcination temperatures. When the calcinations temperature is below 300 °C, MnO2 is still in amorphous state. However, α-MnO2 appears when MnO2 is heated between 400 and 500 °C and transforms to α-Mn2O3 above 500 °C.3 It is reported that if MnO2 was in amorphous state, it is not stable during cycling as the electrode material, which is adverse to the diffusion of electrolyte. The specific capacity of electrodes decreases sharply when calcination temperature exceeds 450 °C, because the specific capacity of α-Mn2O3 is very little. Therefore, 450 °C is adopted as the fitting calcination temperature.
The fitting preparation conditions of Y/ZrO2@MnO2 particles are: the mass of ZrOCl2·8H2O is 0.6171 g, the mole ratio between Y(NO3)3·6H2O and ZrOCl2·8H2O (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) 3
mol) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the adding amount of CTAB 7 mmol L−1, the reaction time 3 h, the calcination temperature 450 °C. Characterizations and electrochemical tests of electrodes prepared under fitting preparation conditions were analyzed and researched as below.
100, the adding amount of CTAB 7 mmol L−1, the reaction time 3 h, the calcination temperature 450 °C. Characterizations and electrochemical tests of electrodes prepared under fitting preparation conditions were analyzed and researched as below.
3.2 Characterizations
The morphology of pure MnO2 particles and Y/ZrO2@MnO2 particles are shown in Fig. 6. SEM images in Fig. 6(A) reveals that the pure MnO2 particles perform sphere morphology with smooth surface; meanwhile they have a particle size about 450 nm. Compared to the SEM image of the pure MnO2 particles, the surface of Y/ZrO2@MnO2 particles becomes relatively rough in Fig. 6(B). These results indicate that MnO2 materials have been indeed coated by Y/ZrO2 coatings. Besides, by comparing the images in Fig. 6(B) and (C), it is proved that the morphology of Y/ZrO2@MnO2 has no obvious changes after 5000 cycles and the inorganic coating shell increases the cycle stability of the electrode.
TEM images in Fig. 7(A)–(C) show that MnO2 particles are successfully by Y/ZrO2 coatings. In these images, deep color regions stand for MnO2 particles and gray sections represent Y/ZrO2 coatings. The thickness of the coating shells is between 20 nm and 50 nm in Fig. 7(A)–(C). This result is congruent with the result of SEM analysis. Moreover, EDX spectrum indicates that the coating shell includes Y and Zr element, which proves that yttrium is successfully doped into the ZrO2 coating shells.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) 3
mol) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the adding amount of CTAB 7 mmol L−1; the reaction time 3 h; the calcination temperature 450 °C.
100, the adding amount of CTAB 7 mmol L−1; the reaction time 3 h; the calcination temperature 450 °C.The XRD patterns for P-MnO2 and Y/ZrO2@MnO2 samples are she own in Fig. 8. In the pattern of the two samples, all diffraction peaks can be indexed to the α-MnO2 according to the JCPDS 44-0141 standard file.25 It can be seen from the Fig. 8 that there are some new diffraction peaks appearing and slight deviation of peak position through comparing the patterns of P-MnO2 and Y/ZrO2@MnO2 samples. The new peaks correspond to the ZrO2 according to the JCPDS 07-0343 standard file.26 The result indicates the MnO2 succeed to be coated by ZrO2. Meanwhile, no extra peaks pertaining to Y are observed, indicating that no significant changes of the structure occurred during the doping process.
3.3 Electrochemical tests
As indicated by SEM and TEM results, Y/ZrO2@MnO2 materials is the potential choice application in energy field. To explore the potential applications in electrochemical energy storage, the Y/ZrO2@MnO2 materials are used to make supercapacitor electrodes, and characterized by galvanostatic charge/discharge test, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). | ||
| Fig. 9 The effect of cycle number on the specific capacity of electrodes: (A) 100 cycles (B) 5000 cycles. | ||
The reason suggests that: the protection of Y-doped ZrO2 coating, which prevents direct contact between MnO2 electrode and electrolyte, reduces partial dissolution of electrode materials in the electrolyte during cycling test. Simultaneously, the expansion and contraction of MnO2 crystal lattice is limited by Y-doped ZrO2 coating, thus the cycle stability of the MnO2 electrode is improved. Alternatively, the conductivity of ZrO2 coating is improved by doping yttrium (Y), which decreases surface resistance of ZrO2 coating on the surface of MnO2 electrode and charge-transfer resistance of the whole electrode.
The electrochemical properties of the two electrodes are evaluated using CV tests in 0.5 mol L−1 Na2SO4 solution at the scan rate of 50 mV s−1. As shown in Fig. 11, the test result of the first cycle is compared with that of 100 cycles under same conditions. For pure MnO2 electrode evaluated in Fig. 11(A), although the shape of the CV curves maintain rectangle, the response current decreases obviously after 100 cycles, which implies that the specific capacity of electrode decreases obviously. Then, to compare with the pure MnO2 electrode, the Y/ZrO2@MnO2 electrode is shown in Fig. 11(B), and the CV curves of Y/ZrO2@MnO2 electrode change little after 100 cycles, which indicates that the response current almost stays the same. Therefore, by comparing the curves of the two electrodes, it is proved that the inorganic coating shell increases the cycle stability of the electrode. This result is congruent with the conclusion of former analysis.
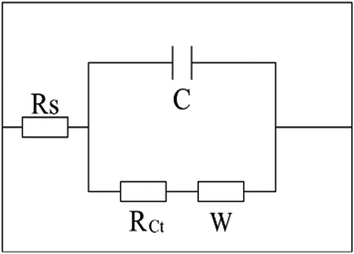
For further investigation, electrodes are described by an equivalent circuit. As seen in Fig. 13, the equivalent circuit is consisted of the electrolyte resistance (Rs), the charge transfer resistance (RCt), the Warburg impedance related to the diffusion in electrolyte (W) and the electrochemical capacitance (C). Based on this equivalent circuit, the values of Rs, RCt and W can be obtained via the software of ZsimpWin, and the corresponding results are listed in Table 1. It can be seen from Table 1, the Rs values of two electrodes are very similar because of the consistent electrolyte. The RCt values of Y-ZrO2@MnO2 electrode are significantly less than the P-MnO2 electrode and the W values of two electrodes are basically the same. These results indicate that the Y-ZrO2 coating reduces the charge-transfer resistance of MnO2 and hardly hinders the diffusion of the electrolyte inside the electrode, which conforms to the Nyquist plots in Fig. 13. The results further demonstrate that the conductivity and the electrochemical properties of the whole MnO2 electrode can be enhanced by Y-doped ZrO2 coating significantly.
| Types of electrode | Rs/Ω cm2 | RCt/Ω cm2 | W/Ω cm2 |
|---|---|---|---|
| P-MnO2 | 0.874 | 1.853 | 0.241 |
| Y-ZrO2@MnO2 | 0.842 | 0.921 | 0.256 |
The EIS of pure MnO2 electrodes during electrochemical cycling is shown in Fig. 14(A). Nyquist plot shows that the radius of depressed arc at high frequencies after 100 cycles is much larger than that after 1 cycle, which implies the resistance of charge transfer process increases with increasing cycle number. The increasing resistance of charge transfer process might be induced by the swells and contracts of MnO2 lattice during charge–discharge,27 which makes the micro-structure collapse and enlarges internal resistance. Finally, the collapse of the microstructure makes the specific capacity decrease sharply. For the low frequencies in Nyquist plot, the slope of the line after 100 cycles is lower than that after 1 cycle, which implies that the diffusion resistance of electrolyte inside electrodes greatly increases after 100 cycles. This is because the collapse of microstructure hinders the diffusion of electrolyte. The curve in Fig. 14(B) shows the admittance plot of pure MnO2 electrodes. The knee frequency for pure MnO2 electrodes is reduced from 8.1 Hz (1 cycle) to 3.7 Hz (100 cycles), implying degraded electrochemical response. According to phase angle–frequency plot in Fig. 14(C), the capacitor response frequency is reduced from 0.12 Hz (1 cycle) to 0.026 Hz (100 cycles). It decreases by 80%, which makes the capacitor response time increases from 8 s to 40 s. As shown in the curves in Fig. 14, the EIS of pure MnO2 electrodes varies greatly after 100 cycles. The resistance of both charge transfer process and electrolyte diffusion process increases, which reduces the electrochemical response ability and increases capacitor response time.
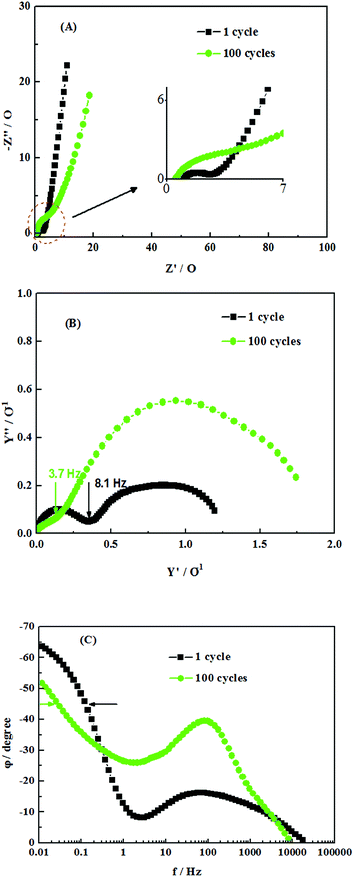 | ||
| Fig. 14 Effects of cycles number on EIS of pure MnO2 electrodes: (A) Nyquist plot (B) admittance plot (C) phase angle–frequency plot. | ||
The effect of cycle numbers on the EIS of Y/ZrO2@MnO2 electrodes is shown in Fig. 15. Nyquist plot in Fig. 15(A) shows that the radius of depressed arc at high frequencies after 100 cycles is similar to that after 1 cycle, which implies the resistance of charge transfer process during electrodes process mainly remains the same with increasing cycle numbers. Although MnO2 lattice continually swells and contracts during charge–discharge process, inorganic coating shells effectively protect the microstructure of electrodes, which enhances the cycle stability of the electrodes. For the straight line at the low frequency range, its slope changes little after 100 cycles, indicating the electrolyte diffusion resistance inside the electrodes hardly changes. Fig. 15(B) shows the admittance plot of Y/ZrO2@MnO2 electrodes. The knee frequency is 2.1 Hz (1 cycle) and 1.9 Hz (100 cycles), which implies the capacitor response ability changes little. According to Fig. 13(C), the capacitor response frequency is 0.12 Hz (1 cycle) and 0.098 Hz (100 cycles), which only decreases by 19%. At the same time, capacitor response time increases from 8 s (1 cycle) to 10 s (100 cycles). The curves in Fig. 13 show that the EIS of Y/ZrO2@MnO2 electrodes changes little after 100 cycles. The resistance of charge transfer process and electrolyte diffusion process remains largely unchanged, which makes the electrochemical response ability keep the same and the capacitor response time increase little. Compared to the EIS of pure MnO2 electrodes, the results prove that the inorganic coating shell increases the cycle stability of the electrode indeed.
4. Conclusions
In this paper, a cost-effective and simple strategy is presented to design and fabricate novel Y/ZrO2@MnO2 material, to improve the cycle stability and conductivity property of electrode for supercapacitor. The fitting preparation conditions of Y/ZrO2@MnO2 particles are: the mass of ZrOCl2·8H2O is 0.6171 g, the mole ratio between Y(NO3)3·6H2O and ZrOCl2·8H2O (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) 3
mol) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the adding amount of CTAB 7 mmol L−1; the reaction time 3 h; the calcination temperature 450 °C. The results of SEM, TEM, EDX and XRD about electrodes prepared under the fitting preparation conditions show that MnO2 materials are indeed coated by Y-doped ZrO2 coatings and Y/ZrO2@MnO2 particles possess a core–shell structure. The results of electrochemical tests show that the Y/ZrO2@MnO2 electrodes display better cycling stability and capacity performance. Therefore, Y-doped ZrO2 coating is the potential choice to improve the cycling stability and conductivity of MnO2 electrode.
100, the adding amount of CTAB 7 mmol L−1; the reaction time 3 h; the calcination temperature 450 °C. The results of SEM, TEM, EDX and XRD about electrodes prepared under the fitting preparation conditions show that MnO2 materials are indeed coated by Y-doped ZrO2 coatings and Y/ZrO2@MnO2 particles possess a core–shell structure. The results of electrochemical tests show that the Y/ZrO2@MnO2 electrodes display better cycling stability and capacity performance. Therefore, Y-doped ZrO2 coating is the potential choice to improve the cycling stability and conductivity of MnO2 electrode.
Acknowledgements
This project is supported by National Natural Science Foundation of China (No. 21076143), by the key technologies R & D program of Tianjin (15ZCZDSF00160), by the Basic Research of Tianjin Municipal Science and Technology Commission (13JCYBJC20100), by Tianjin Municipal Science and Technology Xinghai Program (No. KJXH2014-05), by China Scholarship Council (No. 201406255018).References
- J. R. Miller and P. Simon, Science, 2008, 32, 651–652 CrossRef PubMed.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- G. Jiang, M. Y. Zhang, X. Q. Li and H. Gao, RSC Adv., 2015, 85, 69365–69370 RSC.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- G. H. Yu, X. Xie and L. J. Pan, Nano Energy, 2013, 2, 213–214 CrossRef CAS.
- Y. Hou, Y. W. Cheng and T. Hobson, Nano Lett., 2010, 10, 2727–2733 CrossRef CAS PubMed.
- Y. S. Qudaih, A. A. Elbaset and T. Hiyama, Int. J. Electr. Power Energ. Syst. Eng., 2011, 33, 43–54 CrossRef.
- C. X. Gu, M. Wang, T. Chen, X. W. Lou and C. M. Li, Adv. Energy Mater., 2011, 1, 736–741 CrossRef.
- X. Lu, T. Zhai, X. Zhang, Y. Shen, L. Yuan and B. Hu, Adv. Mater., 2012, 24, 938–944 CrossRef CAS PubMed.
- L. H. Bao, J. F. Zang and X. D. Li, Nano Lett., 2011, 11, 1215–1220 CrossRef CAS PubMed.
- Y. C. Hsieh, K. T. Lee, Y. P. Lin, N. L. Wu and S. W. Donne, J. Power Sources, 2008, 177, 660–664 CrossRef CAS.
- J. K. Chang, M. T. Lee and W. T. Tsai, J. Power Sources, 2007, 166, 590–594 CrossRef CAS.
- S. C. Pang, M. A. Anderson and T. W. Chapman, J. Electrochem. Soc., 2000, 147, 444–450 CrossRef CAS.
- S. C. Pan and M. Anderson, J. Mater. Res., 2000, 15, 2096–2106 CrossRef.
- R. N. Reddy and R. G. Reddy, J. Power Sources, 2003, 124, 330–337 CrossRef CAS.
- X. W. Yu and S. Licht, J. Power Sources, 2007, 173, 1012–1016 CrossRef CAS.
- K. A. Walz, J. R. Szczech, A. N. Suyama, W. E. Suyama, L. C. Stoiber and W. A. Zeltner, et al., J. Electrochem. Soc., 2006, 153, A1102–A1107 CrossRef CAS.
- S. Licht, X. W. Yu and Y. F. Wang, J. Electrochem. Soc., 2008, 155, A1–A7 CrossRef CAS.
- S. Licht, X. W. Yu and D. Zheng, Chem. Commun., 2006, 41, 4341–4343 RSC.
- P. Li, I. W. Chen and J. E. Penner-Hahn, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 10063–10073 CrossRef CAS.
- J. C. Ray, R. K. Pati and P. Pramanik, J. Eur. Ceram. Soc., 2000, 20, 1289–1295 CrossRef CAS.
- A. Ghosh, A. K. Suri and M. Pandey, Mater. Lett., 2006, 60, 1170–1173 CrossRef CAS.
- Y. Q. Zhang, G. D. Zhang and T. D. Du, Electrochim. Acta, 2011, 56, 1159–1163 CrossRef CAS.
- Y. Q. Zhang, X. H. Zhao, S. M. Zhang, G. D. Zhang and S. M. Liu, Appl. Energy, 2012, 99, 265–271 CrossRef CAS.
- H. P. Klug and L. E. Alexander, X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, Wiley, New York, 2nd edn, 1974, pp. 554–557 Search PubMed.
- K. Sachin, B. Snehasis, S. Jitendra and K. O. Animesh, J. Alloys Compd., 2015, 649, 348–356 CrossRef.
- W. F. Wei, X. W. Cui, W. X. Chen and D. G. Ivey, J. Power Sources, 2009, 186, 543–550 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |