Simultaneous removal of NH4+-N and refractory organics through sequential heterogeneous Fenton oxidation process and struvite precipitation: kinetic study
P. Maharaja,
E. Gokul,
N. Prabhakaran,
K. Patchai murugan,
S. Karthikeyan,
R. Boopathy,
S. Swarnalatha and
G. Sekaran*
Environmental Technology Division, Council of Scientific & Industrial Research (CSIR), Central Leather Research Institute (CLRI), Adyar, Chennai 600 020, India. E-mail: ganesansekaran@gmail.com; Fax: +91-44-24452941; Tel: +91-44-24911386 extn 7241
First published on 18th December 2015
Abstract
The aim of the present investigation was to treat wastewater containing a high concentration of NH4+-N by heterogeneous Fenton oxidation of organics and struvite precipitation. The Fenton reagent (Fe2+/H2O2-10 mM/9.8 mM) and nanoporous activated carbon (30 g L−1) as the heterogeneous matrix were used in heterogeneous Fenton oxidation (HFO) process for the destruction of refractory organic compounds present in ammoniacal nitrogen containing wastewater (ANWW). The HFO process was followed by NH4+-N removal from the ANWW as struvite crystals using MgO and Na2HPO4·2H2O. The maximum removal of NH4+-N as struvite was 96% at solution pH 9.0 and at ambient temperature after the destruction of organic compounds by a HFO process. The optimum time for struvite precipitation was 60 min and secondary crystallization time for struvite crystals was 2 h. The removal of organic impurities was confirmed through FT-IR analysis of struvite crystals formed with and without HFO treatment. The thermal stability of struvite crystals was evaluated by TGA and DTA analyses and enthalpy of formation of struvite was determined through DSC analysis. The orthorhombic crystalline nature of the struvite crystals recovered from ANWW and [ANWW]HFO was evaluated using XRD spectroscopy. The surface morphology of precipitated struvite was analysed using scanning electron microscopy.
Introduction
Leather, textiles, pharmaceuticals and chemical industries discharge wastewater containing high concentration of nitrogen in the form of ammonia, nitrite and nitrate.1,2 The direct discharge of high concentrations of ammoniacal nitrogen deteriorates receiving water quality and it also severely damages the local ecology.3 Therefore, it has become necessary to eliminate these inorganic nitrogeneous compounds from the wastewater before they enter into any aquatic systems. The available processes, which possess practical importance, can be broadly classified into two main categories such as biological processes and physicochemical processes. The biological processes for the treatment of wastewater containing NH4+-N include nitrification–denitrification processes and the anammox process.4,5 The excess addition of methanol to the system, the pH maintenance and high hydraulic retention time for the treatment of wastewater containing nitrogen were considered to be the major limitations of the biological treatment processes.6 Therefore, only few processes are being exploited on a commercial scale. The physicochemical processes involve stripping of NH4+-N from the effluent by air or steam; the electrochemical conversion and ion exchange method were also reported for treatment of ammoniacal nitrogen containing wastewater (ANWW).7–10 The major limitations of these physicochemical processes are high capital and operating costs and also the fact that the ammonia stripped out from ANWW contributes to air pollution.11Magnesium ammonium phosphate precipitation (MAP process) is considered to be one of the most effective methods for managing high NH4+-N containing wastewater. MAP is also known as struvite, a crystalline substance containing magnesium, phosphate and ammonium ions at the equimolar ratios that is represented with molecular formula MgNH4PO4·6H2O. Struvite may be regarded as a slow release type of fertilizer.12–15 Moreover, struvite would be the most alternative fertilizer for commercial crops, such as sugar beet, which needs magnesium content.16 The pH plays an important role during the MAP precipitation process. Struvite or MAP may be precipitated at a wide range of alkaline pH values (7.0–11.5), but the suitable pH range was found to be 7.5 to 9.0.17–19 The precipitation of struvite occurs in the presence of magnesium (Mg2+), ammonium (NH4+), and phosphate (PO43−) ions as per eqn (1) as follows:
| Mg2+ + NH4+ + PO43− +6H2O → MgNH4PO4·6H2O | (1) |
According to eqn (1), Mg2+, NH4+, and PO43− are required to be in equimolar quantities to form MgNH4PO4·6H2O. However, certain chemical species present in an aqueous solution consume Mg2+, NH4+ and/or PO43− compounds by isomorphous crystallization. Therefore, precipitation of struvite needed additional Mg2+ and/or PO43− higher than the stoichiometric ratio for the maximum yield.20–22
The struvite crystals recovered from the wastewater stream contained organic compounds as an inclusion and thus, the reuse of struvite crystals has limited applications.17 Microwave irradiation dissociates the struvite into Mg2+, NH4+, and PO43− ions that are recycled again to the influent wastewaters for struvite precipitation that reduces the struvite production cost.23 Therefore, the use of struvite as the fertilizer or as the source of magnesium ions and phosphate ions for the recycling process recovered from the wastewater streams should be in the pure condition to prevent the effect of organics present in the wastewater. Moreover, the presence of organics in wastewater retards the MAP precipitation resulting in poor removal efficiency. Thus, it is essential to eliminate the organic impurities from the wastewater to enhance the NH4+-N removal efficiency and to increase the purity of the struvite crystals.
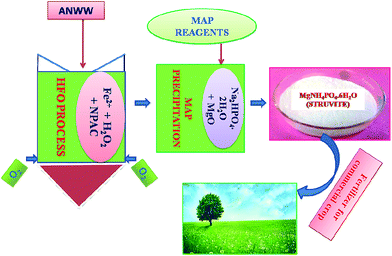
Karthikeyan et al. in 2012 has reported the removal of organic impurities from the NH4+-N containing tannery wastewater by a heterogeneous Fenton oxidation process.24 The hetero Fenton oxidation (HFO) process consists of the catalytic oxidation of organics in wastewater by in situ generated hydroxyl radical, having high oxidation potential (2.8 V), using nanoporous activated carbon as the heterogeneous catalyst. Therefore, this present investigation was focused on treating NH4+-N containing wastewater by integrated heterogeneous Fenton oxidation (HFO) of organics followed by precipitation of ammonium ion as magnesium ammonium phosphate (MAP), which is presented in Fig. 1.
Materials and methods
Materials
All the chemicals used in the present study, such as MgO, Na2HPO4·2H2O, FeSO4·7H2O, hydrogen peroxide (30% v/v), were of analytical grade purchased from Sigma-Aldrich, India.Collection and characterization of wastewater
The ANWW was collected from a chemical industry cluster engaged in manufacturing of pigments, paints, and dyestuffs located in Gujarat, India. The various physicochemical parameters were analysed by following the methods as detailed in standard methods for analysis of water and wastewater.25 The wastewater sample was pretreated to remove the coarse suspended solids by sand filtration. The characteristics of raw wastewater before and after sand filtration are presented in Table 1.| Parameters | Initial wastewater | Wastewater after sand filtration |
|---|---|---|
| a All the values except pH and BOD5/COD are expressed in mg L−1. | ||
| pH | 0.82 | 0.87 |
| Chemical oxygen demand, COD | 8720 | 8530 |
| Biochemical oxygen demand, BOD5 | 2420 | 2390 |
| BOD5/COD | 0.27 | 0.273 |
| Total organic carbon, TOC | 1662 | 1658 |
| Ammoniacal nitrogen, NH4+-N | 1915 | 1905 |
| Total Kjeldhal nitrogen, TKN | 1982 | 1977 |
| Total solids | 9647 | 9598 |
| Total dissolved solids | 9420 | 9412 |
| Total suspended solids | 227 | 106 |
Preparation of nanoporous activated carbon (NPAC)
The heterogeneous catalyst, NPAC was prepared from rice husk an agricultural solid waste. The procedure followed for the preparation of nanoporous activated carbon (NPAC) was described in detail by the authors in their previous publication.26 NPAC was prepared by two sequential steps such as pre-carbonization and chemical activation. The washed rice husk was packed in an air-tight graphite crucible and pre-carbonized at 400 °C for 4 h. The pre-carbonized material was activated using ortho phosphoric acid (H3PO4) at 800 °C at a 5 °C min−1 heating rate. The activated carbon was further oxidized using 5 M nitric acid (HNO3) at 60 °C for 3 h. The NPAC, upon oxidation with nitric acid was imparted with oxygenated functional groups, such as –COOH, –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, on the surface of the matrix. The oxidized NPAC was washed with acid/alkali to get neutral condition. The oxidized NPAC was dried at 110 °C in a hot air oven for four hours to drive away the moisture and it was designated as NPAC.
O, on the surface of the matrix. The oxidized NPAC was washed with acid/alkali to get neutral condition. The oxidized NPAC was dried at 110 °C in a hot air oven for four hours to drive away the moisture and it was designated as NPAC.
Characterization of NPAC catalyst and MAP product
NPAC was characterized for surface area, pore volume and pore size distribution by an automatic adsorption instrument (Quantachrome Corporation, USA). The elemental composition (carbon, hydrogen and nitrogen content) of the NPAC was determined using Vario MICRO CHNSO 15091002 (Carlo–Erba analyser, Germany). FT-IR spectroscopy (Perkin-Elmer, USA) was used for investigating the surface functional groups in MAP. Spectral analysis was recorded in the range of 4000–400 cm−1. MAP samples (0.1 g) were homogenised with spectroscopy grade KBr (1 g, Merk, Darmstadt, Germany) in a mortar. The crystalline nature of MAP precipitate was determined by X-ray diffraction (XRD) analysis in a Rich Siefert 3000 diffractometer using Cu-Kα1 radiation (λ = 0.1541 nm). The morphology of MAP samples recovered from ANWW and [ANWW]HFO were evaluated by SEM analysis. The SEM analysis was performed using a scanning device attached to a JEOL JM 5600 electron microscope at 20 kV (JEOL, Japan) accelerating voltage with a 5–6 nm electron beam. Thermo gravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis were carried out from 30 to 800 °C with a 10 °C min−1 temperature gradient under a nitrogen atmosphere and scans were recorded using a TGA Q50 (V20.6 Build 31, USA).Characteristics of NPAC
The NPAC was selected as a heterogeneous catalyst for the oxidation of dissolved organics in wastewater for its endowed characteristics such as surface area, 379 m2 g−1; pore volume, 0.188 cm3 g−1; pore diameter, 39.36 Å; C, 41.5%; H, 2.85%; N, 0.7%; and free electron density, 1.60 × 1022 spins per g. The maximum reflectance measured at λ800 nm in a UV-visible spectrophotometer correlates with the energy gap (Eg) of 1.55 eV for NPAC, which explains the extrinsic semiconductor property of NPAC.25Characterization of ammoniacal nitrogen containing wastewater (ANWW)
The ANWW and [ANWW]HFO samples were characterised for Chemical Oxygen Demand (COD), Biochemical Oxygen Demand (BOD5) as per standard methods described in APHA (1995). The NH4+-N and Total Kjeldhal Nitrogen (TKN) of wastewater samples were determined by the Kjeldhal distillation method using a Buchi distillation unit K-350 (BUCHI, Switzerland). The pH of the solution was measured using a precalibrated pH meter (Systronics 620, India). Total organic carbon (TOC) was analysed by a TOC analyser (SHIMADZU Model no: SHIMADZU CORP 00291, India). The TOC analyzer was calibrated using potassium hydrogen phthalate at 1000 ppm as a standard TOC solution.Heterogeneous Fenton oxidation (HFO) process
The heterogeneous Fenton oxidation process was used for treating ANWW. In this study, Fe2+/H2O2 was selected as the Fenton reagent and NPAC (30 g L−1) as the heterogeneous catalyst for the destruction of organic compounds in wastewater.27,28 The experiment was carried out by taking one litre of ANWW sample in a fluidized bed reactor (10.2 cm × 10.2 cm × 22.4 cm dimensions) with a 1.4 L total volume. The Fenton reagent, a mixture of hydrogen peroxide H2O2 (9.8 mM) and FeSO4·7H2O (10 mM), was used for the oxidation of refractory organics in wastewater. Hydroxyl radicals are generated from Fenton reagent (ferrous ion and hydrogen peroxide) as per the following equations:| H2O2 + Fe2+ → ˙OH + OH− + Fe3+ | (2) |
| Fe3+ + H2O2 → Fe2+ + HO2˙ + H+ | (3) |
The hydroxyl radical is also generated from NPAC and molecular oxygen in the HFO process. The electron transfer from electron rich NPAC to molecular oxygen generates reactive oxygen species (eqn (4)), which is considered to be the primary step in formation of hydroxyl radicals with NPAC.26
| NPAC(e−cb) + O2 → NPAC(O2˙)ads | (4) |
The formation of hydroxyl radicals from the reactive oxygen species in the adsorbed state occurs as explained in eqn (5) and (6) as follows:
| NPAC(h+vb) + H2O → NPAC(˙OH)ads + H+aq | (5) |
| NPAC(O2˙)ads + H+aq → NPAC(2˙OH)ads | (6) |
The hydroxyl radicals react with organic pollutants in wastewater in a short time and convert them into smaller and biodegradable organic compounds. At the end of the complete chemical reaction, the system has carbon dioxide, water and fragmented organic compounds with low molecular weights remaining according to eqn (7) as follows:
| ˙OH + refractory organic compounds → oxidized compounds + CO2 + H2O | (7) |
Initially, the ANWW was filtered at its native pH 0.82 through a sand filter to remove suspended solids. The HFO process was carried out by taking 1 litre of sand filtered ANWW at its pH, 0.87. Fenton reagent (H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2+ at 9.8 mM
Fe2+ at 9.8 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mM) and NPAC (30 g L−1) were added to the fluidized bed reactor and the contents were fluidized by compressed air at 2.0 kg cm−2 pressure supplied through an air sparger provided at the bottom of the reactor. The oxidation of organics in ANWW was carried out under ambient conditions. The samples were periodically collected at different time intervals (2, 4, 6, 8 and 10 h) from the HFO reactor for the characterisation of BOD and COD. The heterogeneous Fenton oxidised ammoniacal nitrogen containing wastewater was named [ANWW]HFO.
10 mM) and NPAC (30 g L−1) were added to the fluidized bed reactor and the contents were fluidized by compressed air at 2.0 kg cm−2 pressure supplied through an air sparger provided at the bottom of the reactor. The oxidation of organics in ANWW was carried out under ambient conditions. The samples were periodically collected at different time intervals (2, 4, 6, 8 and 10 h) from the HFO reactor for the characterisation of BOD and COD. The heterogeneous Fenton oxidised ammoniacal nitrogen containing wastewater was named [ANWW]HFO.
MAP precipitation
The precipitation of NH4+-N from ANWW as struvite was carried out by adjusting the ANWW and [ANWW]HFO pH to 7.0 using sodium hydroxide (10% w/v). The sequential addition of MAP reagents was in the order of phosphate source (Na2HPO4·2H2O) followed by magnesium oxide (MgO) and the solution was agitated at 50 rpm for 60 min using a magnetic stirrer. The secondary crystallization of struvite was allowed for 2 h and the NH4+-N free wastewater was separated by filtration using a glass microfiber filter paper (0.45 μm, GF/A). The experiment was repeated at different reaction time periods, such as 60, 90 and 120 min, as the rate of agitation and the solution pH play a significant role in the formation of nuclei in struvite precipitation.17,29 The supernatant solution, after settling the struvite crystals, was collected and characterized for NH4+-N to evaluate the removal efficiency. The molar ratio of MAP reagents (MgO and Na2HPO4·2H2O) was varied for the optimization of maximum removal of NH4+-N present in the wastewater. The integrated heterogeneous Fenton oxidation and struvite precipitation process is shown in Fig. 2.Results and discussion
Treatment efficiency and formation of struvite precipitate
The removal efficiency of NH4+-N and purity of the recovered struvite in the integrated process were evaluated.Heterogeneous Fenton oxidation (HFO) process
Kinetic study for HFO process:
The kinetics on the removal of organics by HFO process was studied by applying pseudo first order and pseudo second order kinetic models as expressed in eqn (8) and (9) as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CODt/COD0 = −k1t, CODt/COD0 = −k1t,
| (8) |
| 1/CODt = 1/COD0 + k2t, | (9) |
The kinetic study of the HFO process was carried out and the results are shown in Fig. 4(a) and (b). The results illustrate that COD removal by the HFO process best obeyed the pseudo second order rate kinetic model with a regression coefficient value of 0.997 and χ2 value of 0.0023, as shown in Table 2. This indicated that the rate of the reaction depended upon the Fenton reagent and NPAC/O2 for the removal of refractory organics present in ANWW during HFO process.
 | ||
| Fig. 4 (a) Pseudo first order rate kinetic model for the COD removal in HFO process. (b) Pseudo second order rate kinetic model for the COD removal in the HFO process. | ||
| COD removal | |||||
|---|---|---|---|---|---|
| Pseudo first order | Pseudo second order | ||||
| k1 (min−1) | R2 | χ2 | k2 (L mol−1 min−1) | R2 | χ2 |
| 0.088 | 0.809 | 0.078 | 2.575 × 10−5 | 0.997 | 0.0023 |
An increase in the solution pH after addition the MAP reagents increased the crystal growth by supersaturation.30 In general, the formation of struvite crystals depends upon the reactant concentration and solution pH. As mentioned above, struvite or MAP may be precipitated in the wide range of alkaline pH (7.0–11.5), but the most suitable pH range was found to be 7.5 to 9.0.17–19 This may be due to decrease in solubility of struvite with increasing solution pH. In the present investigation, the struvite precipitation was performed at pH 7.0 after the HFO process. The struvite precipitation was initiated by adding Na2HPO4·2H2O at the required molar ratio (1.0 to 1.5), and it was followed by adding another MAP reagent, MgO at the required molar ratio (1.0 to 1.5). Table 3 shows that increasing the molar ratio of MAP reagents from 1.0 to 1.5 increased the solution pH significantly.
Stoichiometric molar ratio (NH4+-N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg2+ Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO43−) PO43−) |
Solution pH after the addition of Na2HPO4·2H2O | Solution pH after the addition of MgO |
|---|---|---|
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
5.92 | 8.33 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
5.87 | 8.31 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
5.74 | 8.28 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
5.68 | 8.35 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
5.62 | 8.32 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
5.58 | 8.32 |
After adding two MAP reagents, the solution pH was adjusted to different alkaline pH values (8.5 to 10.5) using sodium hydroxide (10% w/v) for the secondary struvite crystallization. The results revealed that the maximum removal of NH4+-N was observed at pH 9.0 and thereafter the removal efficiency of NH4+-N was not increased significantly. Therefore, the optimum solution pH for the maximum struvite precipitation was fixed at 9.0. The secondary struvite crystallization enhanced the NH4+-N removal by 20–25% and it completed the struvite formation process.
MAP precipitation after the organic removal by HFO process
The MAP precipitation was carried out in the following four steps:(i) The destruction of organic compounds by a heterogeneous Fenton oxidation process by the Fenton reagent (Fe2+/H2O2) in the presence of nanoporous activated carbon (NPAC) as the heterogeneous catalyst.
(ii) The pH was increased to 7.0 using sodium hydroxide (10% w/v).
(iii) MAP precipitation was carried out using precipitating reagents (MgO and Na2HPO4·2H2O).
(iv) Secondary struvite crystallization by increasing the pH up to 9 using sodium hydroxide (10% w/v).
The HFO process destructs the organic pollutants in the wastewater using hydroxyl radicals generated from Fenton reagent and from NPAC/O2 at its working condition. The NPAC plays an important role in producing hydroxyl radicals and subsequent oxidation of organic compounds in wastewater.28 The nanoporous network structure present in NPAC adsorbs and destructs organic molecules in the wastewater by in situ generated hydroxyl radicals.27 In the present investigation, the COD removal was in the range of 52–55% due to the presence of refractory organic chemicals and high TDS or may be due to partial oxidation of the organics present in wastewater.
The struvite crystals recovered from [ANWW]HFO, named struvite I, was found to have higher purity than the struvite crystals recovered from ANWW, which are named struvite II. This confirmed that the integrated HFO process enabled the recovery of struvite crystals with high purity. After HFO treatment the pH of the solution was adjusted to 7.0 using sodium hydroxide (10% w/v). The precipitation of struvite was optimized by varying the concentration of MAP reagents (MgO and Na2HPO4·2H2O) at the stoichiometric molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, 1
1.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, 1
1.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, 1
1.3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 and 1
1.4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 at ambient conditions with respect to Mg2+
1.5 at ambient conditions with respect to Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4+
NH4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO43− composition. Then, the pH of the solution was increased up to 9 using sodium hydroxide (10% w/v) for secondary crystallization of struvite crystals.
PO43− composition. Then, the pH of the solution was increased up to 9 using sodium hydroxide (10% w/v) for secondary crystallization of struvite crystals.
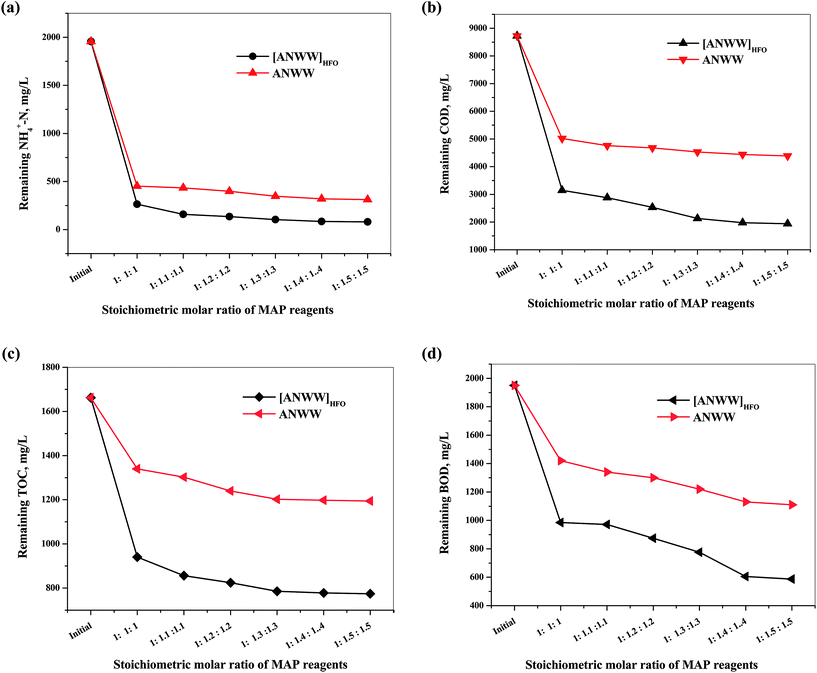
Fig. 5(a–d) shows that the NH4+-N was removed by 86.5% to 96.4% along with COD reduction by 63.8% to 78.2%, BOD by 49.4% to 69.8% and TOC by 51.9% to 54.5% during struvite precipitation from [ANWW]HFO. The percentage removal of NH4+-N increased with increasing the molar ratio of precipitating reagents until the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 stoichiometric molar ratio was reached. Many researchers have reported that the formation of struvite occurs at equimolar concentrations (1
1.4 stoichiometric molar ratio was reached. Many researchers have reported that the formation of struvite occurs at equimolar concentrations (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Mg2+, NH4+, and PO43− species. The consumption of the precipitating reagents higher than the stoichiometric molar ratio of struvite formation was due to the presence of other ionic reactants. Further increase in the ratio did not influence on the reduction of NH4+-N. Therefore, the optimum stoichiometric molar ratio was selected as 1
1) of Mg2+, NH4+, and PO43− species. The consumption of the precipitating reagents higher than the stoichiometric molar ratio of struvite formation was due to the presence of other ionic reactants. Further increase in the ratio did not influence on the reduction of NH4+-N. Therefore, the optimum stoichiometric molar ratio was selected as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 for maximum removal of NH4+-N. The reduction percentage did not increase as noticeably after increasing the stoichiometric molar ratio above 1
1.4 for maximum removal of NH4+-N. The reduction percentage did not increase as noticeably after increasing the stoichiometric molar ratio above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5.
1.5.
MAP precipitation in the absence of HFO process
This study was carried out by the following three steps:(i) Adjusting the initial ANWW pH to 7.0 using sodium hydroxide (10% w/v).
(ii) MAP precipitation was carried out using Na2HPO4·2H2O and MgO.
(iii) Secondary struvite crystallization initiated by increasing the pH to 9 using sodium hydroxide (10% w/v).
The precipitation of struvite crystals using precipitating reagents (MgO and Na2HPO4·2H2O) at different stoichiometric molar ratios of Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4+
NH4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO43− such as 1
PO43− such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, 1
1.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, 1
1.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, 1
1.3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 and 1
1.4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 at ambient conditions. Then, the pH of the solution was increased up to 9 for the secondary crystallization of struvite crystals.
1.5 at ambient conditions. Then, the pH of the solution was increased up to 9 for the secondary crystallization of struvite crystals.
The Fig. 5(a–d) shows that the overall removal of efficiency of NH4+-N was found to be 76.9% to 84.1% along with the reduction in COD by 42.4% to 49.6%, BOD by 27.4% to 43.0% and TOC by 19.3% to 23.1%. The percentage removal of NH4+-N and other pollutants increased with increase in concentration of precipitating reagents (MgO and Na2HPO4·2H2O) up to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and thereafter the removal of organic content remained the same.
1.5 and thereafter the removal of organic content remained the same.
Kinetic study on struvite precipitation after heterogeneous Fenton oxidation of organics in wastewater
The kinetics on the formation of struvite crystals was studied by applying pseudo-first order and pseudo-second order kinetic models as expressed in eqn (10) and (11) as follows,
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) {[NH4+-N]t/[NH4+-N]0} = −k1t {[NH4+-N]t/[NH4+-N]0} = −k1t
| (10) |
| 1/[NH4+-N]t = 1/[NH4+-N]0 + k2t | (11) |
The results of a kinetic study on struvite precipitation after HFO process are presented in Fig. 6(a) and (b). The results illustrate that precipitation of struvite best obeyed a second order rate kinetic model and had a 0.987 regression coefficient and 0.0026χ2, as shown in Table 4. Ohlinger et al.29 studied the MAP process for phosphate removal from anaerobic lagoon effluent and it was reported that the formation of struvite obeyed a first order kinetic model with a 4.2 h−1 rate constant. Nelson et al.14 also reported the first order kinetics with a 12.3 h−1 rate constant (at pH 9.0) for phosphorus removal from anaerobic swine lagoon effluent as struvite. Turker et al.31 reported that the formation of struvite follows the second order rate kinetics for removing ammonia from anaerobic digester effluents. However, in the present investigation, struvite precipitation was limited by the concentrating the MAP reagents; therefore, the kinetics for removing NH4+-N followed a pseudo-second order rate kinetic model. The calculated pseudo-second order rate constant was found to be 0.365 litre mol−1 min−1.
 | ||
| Fig. 6 (a) Pseudo-first order rate kinetic model for NH4+-N removal as struvite crystals. (b) Pseudo-second order rate kinetic model for NH4+-N removal as struvite crystals. | ||
| Ammoniacal nitrogen removal | |||||
|---|---|---|---|---|---|
| Pseudo first order | Pseudo second order | ||||
| k1 (min−1) | R2 | χ2 | k2 (L mol−1 min−1) | R2 | χ2 |
| 0.001 | 0.965 | 0.028 | 0.365 | 0.987 | 0.0026 |
Characterization of recovered struvite by instrumentation techniques
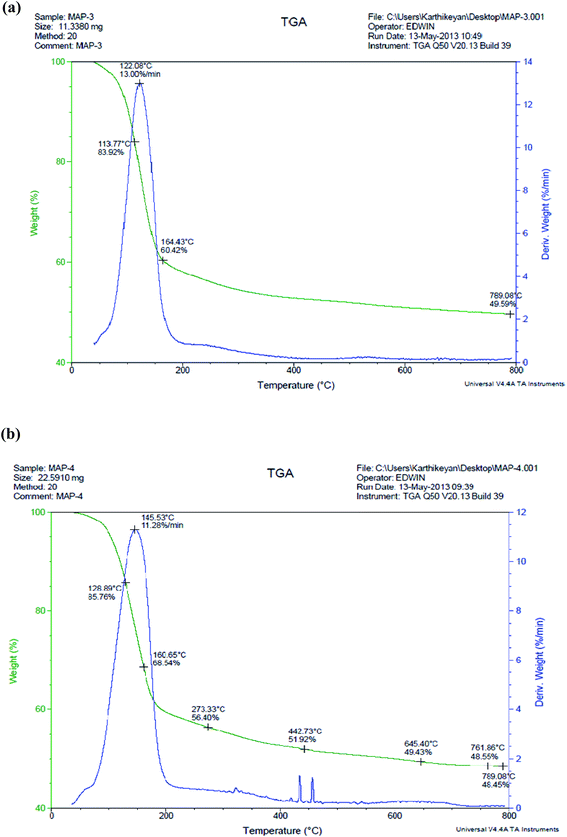 | ||
| Fig. 7 (a) TGA for struvite crystal I recovered from [ANWW]HFO. (b) TGA for struvite crystal II recovered from ANWW. | ||
The activation energy (Ea) was calculated from TGA thermograms of struvite-I and struvite-II by using the following equation,
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [(dw/dt/w)] = ln [(dw/dt/w)] = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/2.303RT A − Ea/2.303RT
| (12) |
The DTA analysis showed a sharp endothermic peak at 122.08 °C that may be attributed to the release of water of crystallization along with NH4+-N. The decomposition at this stage had a 13% weight loss. Thus, the sharpness of the endothermic peak in the DTA analysis may be attributed to the good crystallinity of the struvite crystal. The sharp endothermic peak at 145.53 °C for the struvite II crystal, as shown in Fig. 7(b), is assigned to an 11.28% weight loss. Some minor endothermic peaks observed at 325 °C and 400–450 °C, may be attributed to the presence of organic impurities. These peaks are missing in struvite I, as shown in Fig. 7(a). The DTA study concludes the presence of a good degree of crystallinity in the struvite crystals I and II, and the purity of the struvite was enhanced by the HFO process.
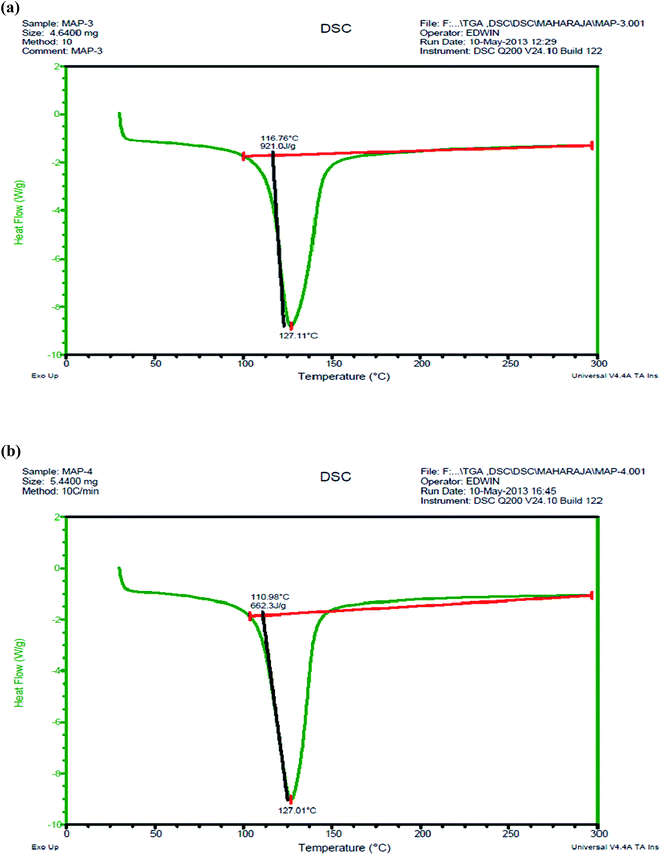
The DSC thermograms for struvite crystals I and II are shown in Fig. 8(a) and (b). The struvite I and struvite II crystals showed the characteristic exothermic peak at 127.01 °C, which may be attributed to the elimination of water of crystallization and ammonia molecules during struvite decomposition.
FT-IR analysis of struvite crystals formed from ANWW and [ANWW]HFO
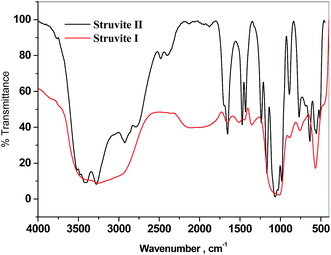
The recovered struvite crystals were characterised for the identification of functional groups by FT-IR analysis. Fig. 9 shows the FT-IR spectra of struvite crystals formed from ANWW and [ANWW]HFO. In Fig. 9, the stretching absorption peak observed at 3249.7 cm−1 confirms the presence of the N–H functional group and the absorption peak at 1657.0 cm−1 is attributed to the N–H bending vibration. The absorption peaks observed at 1513.4 cm−1 and 1354.3 cm−1 are due to the presence of N–H asymmetric stretching vibration of the ammonium ion. The absorption peak at 567.3 cm−1 may be attributed to metal–oxygen bond. The absorption peak at 2105.19 cm−1 is due to the H–O–H stretching vibration of a cluster of water molecules. The absorption peak at 1016.8 cm−1 is due to the presence of a stretching vibration of ionic phosphate (O–P–O) stretching. The absorption peaks at 760.1 cm−1 and 887.6 cm−1 are due to the rocking of N–H bond vibrations. The struvite crystal II formed from ANWW showed more peaks in the aromatic region of the spectrum. This may be attributed to the presence of organic compounds along with struvite components, whereas such peaks are missing in the struvite I.35–37X-ray diffraction and scanning electron microscopic analysis of struvite
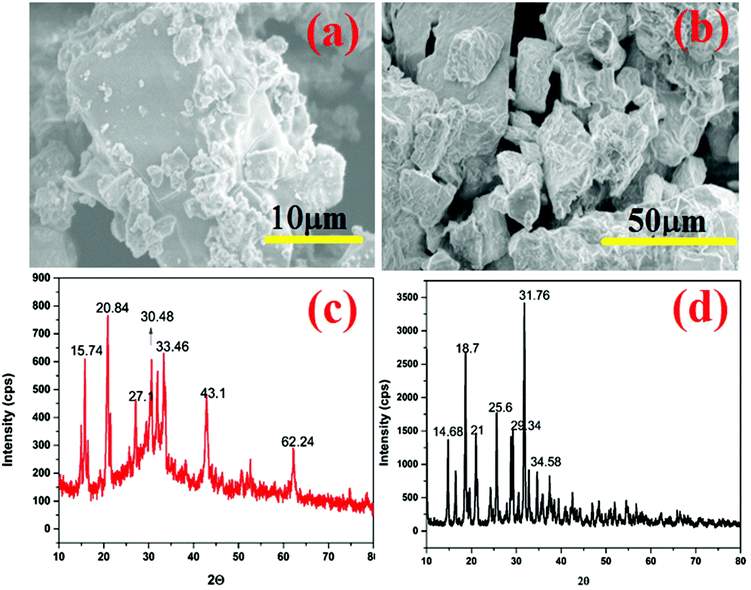
XRD and SEM analyses were performed for the recovered struvite crystals to identify their crystalline nature and to capture their morphology. Fig. 10(a) and (b) show the SEM images of recovered struvite I and struvite II crystals. It was found that the struvite crystal II belongs to the orthorhombic space lattice system with a ∼100 μm average size. The struvite crystal I showed a coffin shape with ∼5 μm average size. The difference in size of struvite crystals I and II may be due to the presence of organic impurities. The larger size of struvite crystal II is due to the agglomeration of struvite crystals with organic molecules during the precipitation process from ANWW. Fig. 10(c) and (d) show the XRD patterns of struvite crystals I and II. The 2θ values observed with the reference of JCPDS (Card no. 1-077-2303)38 indicate the characteristic orthorhombic crystalline planes in both the recovered struvite crystals I and II. However, in struvite crystal II, noise peaks are observed that indicates the inclusion of organic impurities within the crystal lattice system. However, the XRD spectrum showed less noise peaks in struvite I, which may be due to the removal of organic pollutants by the HFO process.17Conclusions
The high concentrated NH4+-N containing wastewater discharged from chemical industry was treated by a HFO process using ˙OH generated from Fe2+/H2O2 and NPAC/O2 for destroying organic compounds in wastewater. The maximum percentage removal of NH4+-N was 94–96% in the MAP process after removing 78% to 86% of COD, BOD by 69% to 75% and TOC by 64% to 70% in HFO process. The molar ratio of NH4-N+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg2+
Mg2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO43− for the maximum yield of struvite precipitation was found to be 1
PO43− for the maximum yield of struvite precipitation was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4.
1.4.
The instrumental analyses, such as TGA, FT-IR and XRD, confirmed that the recovered crystals were struvite and the pure form of struvite was recovered after following HFO process. The precipitation of struvite followed a pseudo second order kinetic model with kinetic constant, 0.365 L mol−1 min−1.
Abbreviations
| COD | Chemical oxygen demand |
| BOD | Biochemical oxygen demand |
| APHA | American public health association |
| TOC | Total organic carbon |
| TKN | Total Kjeldhal nitrogen |
| TGA | Thermo gravimetric analysis |
| DSC | Differential scanning colorimetry |
| DTA | Differential thermal analysis |
| XRD | X-Ray diffraction analysis |
| SEM | Scanning electron microscopy |
| FT-IR | Fourier transform infra red spectroscopy |
| UV-vis | Ultraviolet visible spectroscopy |
| MAP | Magnesium ammonium phosphate |
| NPAC | Nanoporous activated carbon |
| AOP | Advanced oxidation processes |
| HFO | Heterogeneous Fenton oxidation |
| HRT | Hydraulic retention time |
| ANWW | Ammoniacal nitrogen containing wastewater |
| [ANWW]HFO | Ammoniacal nitrogen containing wastewater after heterogeneous Fenton oxidation process |
Acknowledgements
The authors acknowledge CSIR-CLRI, India for granting financial support from STRAIT Project (CSC 0201) to carry out this study.References
- G. E. Diwani, S. E. Rafie, N. N. E. Ibiari and H. I. El-Aila, Desalination, 2007, 214, 200–214 CrossRef.
- O. Tunay, I. Kabdasli, D. Orhon and S. Kolcak, Water Sci. Technol., 1997, 36, 225–228 CrossRef CAS.
- J. N. Galloway, W. H. Schlesinger, H. Levy, A. Michaels and J. L. Schnoor, Global Biogeochem. Cycles, 1995, 9, 235–252 CrossRef CAS.
- U. Welander, T. Henrysson and T. Welander, Water Res., 1998, 32, 1564–1570 CrossRef CAS.
- B. Molinuevo, M. C. García, D. Karakashev and I. Angelidaki, Bioresour. Technol., 2009, 100, 2171–2175 CrossRef CAS PubMed.
- P. Nigam, G. Armour, I. M. Banat, D. Singh and R. Marchant, Biotechnology, 2000, 72, 219–226 CrossRef CAS.
- A. Bonmati and X. Flotats, Waste Manage., 2003, 23, 261–272 CrossRef CAS PubMed.
- K. W. Kim, Y. J. Kim, I. T. Kim, G. I. Park and E. H. Lee, Water Res., 2006, 40, 1431–1441 CrossRef CAS PubMed.
- B. Y. Jeong, S. H. Song, K. W. Baek, I. H. Cho and T. S. Hwang, Polymer, 2006, 30, 486–491 CAS.
- Y. H. Liu, J. H. Kwag, J. H. Kim and C. S. Ra, Desalination, 2011, 277, 364–369 CrossRef CAS.
- H. Siegrist, Water Sci. Technol., 1996, 34(1–2), 399–406 CrossRef CAS.
- R. Gonzalez Poncer and D. M. E. Garcialopez, Agrochimica, 2007, 51, 301–308 Search PubMed.
- K. Yetilmezsoy and Z. S. Zengin, J. Hazard. Mater., 2009, 166, 260–269 CrossRef CAS PubMed.
- N. O. Nelson, Masters thesis, Dept. of Soil Science, North Carolina State University, Raleigh, NC, USA, 2000.
- G. K. Ghosh, K. S. Mohan and A. K. Sarkar, Nutr. Cycling Agroecosyst., 1996, 46, 71–79 CrossRef.
- M. R. Gaterell, R. Gay, R. Wilson, R. J. Gochin and J. N. Lester, Environ. Technol., 2000, 21, 1067–1084 CrossRef CAS.
- X. D. Hao, C. C. Wang, L. Lan and M. C. M. Van Loosdrecht, Water Sci. Technol., 2008, 58, 1687–1692 CrossRef PubMed.
- N. C. Bouropoulos and P. G. Koutsoukos, J. Cryst. Growth, 2000, 213, 381–388 CrossRef CAS.
- M. M. Rahman, Y. H. Liu, J. H. Kwag and C. S. Ra, J. Hazard. Mater., 2011, 186, 2026–2030 CrossRef CAS PubMed.
- D. G. Chirmuley, water: J. Aust. water Assoc., 1994,(4), 21–23 CAS.
- M. Turker and I. Celen, J. Eng. Environ. Sci., 2010, 34, 39–48 Search PubMed.
- I. Stratful, M. D. Scrimshaw and J. N. Lester, Water Res., 2001, 35, 4191–4199 CrossRef CAS PubMed.
- J. H. Cho, J. E. Lee and C. S. Ra, J. Anim. Sci. Technol., 2009, 51, 337–342 CrossRef.
- S. Karthikeyan, M. Ezhil Priya, R. Boopathy, M. Velan, A. B. Mandal and G. Sekaran, Environ. Sci. Pollut. Res., 2012, 19, 1828–1840 CrossRef CAS PubMed.
- APHA, AWWA, WEF, Standard methods for the examination of water and wastewater, 1998, 20th edn.
- S. Kathikeyan and G. Sekaran, Phys. Chem. Chem. Phys., 2014, 16, 3924–3933 RSC.
- E. M. Siedlecka and P. Stepnowski, Environ. Sci. Pollut. Res., 2009, 16, 453–458 CrossRef CAS PubMed.
- S. Karthikeyan, A. Titus, A. Gnanamani, A. B. Mandal and G. Sekaran, Desalination, 2011, 281, 438–445 CrossRef CAS.
- K. N. Ohlinger, T. M. Young and E. D. Schroeder, J. Environ. Eng., 1999, 125, 730–737 CrossRef CAS.
- K. S. Le Corre, E. Valsami-Jones, P. Hobbs and S. A. Parsons, Crit. Rev. Environ. Sci. Technol., 2009, 39, 433–477 CrossRef CAS.
- M. Turker and I. Celen, Bioresour. Technol., 2007, 98, 1529–1534 CrossRef PubMed.
- C. Wang, X. Hao, G. Guo and M. C. M. Van Loosdrecht, Chem. Eng. J., 2010, 159, 280–283 CrossRef CAS.
- M. I. H. Bhuiyan, D. S. Mavinic and F. A. Koch, Chemosphere, 2008, 70, 1347–1356 CrossRef CAS PubMed.
- R. L. Frost, M. L. Weier and K. L. Erickson, J. Therm. Anal. Calorim., 2004, 76, 1025–1033 CrossRef CAS.
- G. Kurtulus and A. C. Tas, Mater. Lett., 2011, 65, 2883–2886 CrossRef CAS.
- B. Soptrajanov, V. Stefov, H. D. Lutz and B. Engelen, J. Mol. Struct., 2005, 752, 60–67 CrossRef.
- B. Soptrajanov, V. Stefov, H. D. Lutz and B. S. Engelen, in Infrared and Raman spectra of magnesium ammonium phosphate hexahydrate (Struvite) and its isomorphous analogues, ed. E. C. Faulques, D. L. Perry and A. V. Yeremenko, NATO Science Series II: Mathematics, Physics and Chemistry: Spectroscopy of Emerging Materials, Springer, Netherlands, 2004, vol. 165, pp. 299–308 Search PubMed.
- E. L. Foletto, W. R. B. D. Santos, M. A. Mazutti, S. L. Jahn and A. Gundel, Mater. Res., 2013, 16, 242–245 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |