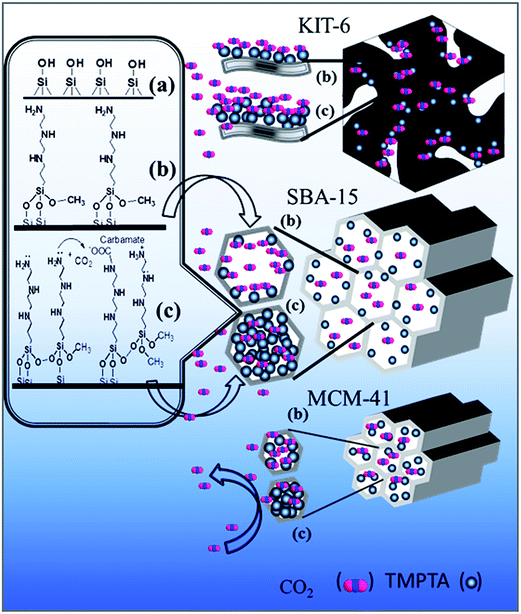
N1-(3-Trimethoxysilylpropyl)diethylenetriamine grafted KIT-6 for CO2/N2 selective separation†
Rupak Kishor* and
Aloke Kumar Ghoshal
Department of Chemical Engineering, Indian Institute of Technology Guwahati, Guwahati-781039, Assam, India. E-mail: rupak.k@iitg.ernet.in
First published on 17th December 2015
Abstract
In the present study N1-(3-trimethoxysilylpropyl)diethylenetriamine was grafted on various ordered and commonly used mesoporous silica namely MCM-41 (2.2 nm), SBA-15 (6.6 nm) and KIT-6 (6.6 nm) in both anhydrous and aqueous conditions for CO2/N2 adsorption. The structural and physical properties before and after grafting were analyzed by nitrogen adsorption/desorption, X-ray diffraction and electron microscopy techniques. The uptake capacities of three-dimensional K30T and WK30T were 1.89 and 2.59 mol CO2 per kg of adsorbent, respectively at 30 °C which are significantly higher than MCM-41 and SBA-15 based adsorbents. Analysis of the enthalpy of CO2 adsorption, confirmed the adsorption in amine functionalized adsorbents by both chemical and physical interactions. Outstanding equilibrium CO2/N2 selectivity of functionalized KIT-6 over MCM-41 and SBA-15 opens up its practical applicability through a stable performance in several adsorptions/desorption cycles.
1. Introduction
Increasing trends of CO2 concentration in the atmosphere are creating alarming conditions in the form of global warming across the globe. Especially in last few decades, the rate of CO2 emission in the form of flue gas has drastically increased from large anthropogenic sources, particularly coal based thermal power plants. This is possibly due to industrialization and growth in energy demand across the globe.1,2 As a result, the atmospheric CO2 concentration increased from ∼315 ppm in 1958 to ∼400 ppm in 2015. Higher CO2 concentrations have led to several environmental issues and therefore, reducing CO2 emissions from the large anthropogenic sources is urgently required.In order to control the CO2 concentration in the atmosphere, carbon capture sequestration and utilization (CCSU) is a promising technology and is becoming indispensable to develop an efficient and cost effective separation process.3 Basic monoethanolamine (MEA), diethanolamine (DEA) and mixed amines based absorption is in commercial operation throughout the world.3,4 But, solid sorbent based separation process could be a potential alternative and a lot of innovative work has been carried out to develop highly CO2/N2 selective adsorbents. CO2 from the anthropogenic source may be separated by a verity of solid physisorbents like zeolite,5 porous carbon,6,7 metal–organic frameworks (MOFs),3,8–12 covalent organic frameworks (COFs),13 polymer14 and chemisorbents like alkali functionalized mesoporous silica and metal carbonates.15–29 Microporous metal–organic frameworks shows very high sorption capacities and selectivity,8–12 but susceptibility to moisture8 demanding for further research for better alternatives. Alkali based metal carbonate (M2CO3, where M = Mg, Na, Cu, Zn etc.) and amine functionalized ordered mesoporous silica (OMSs) based regenerable solid sorbent are good alternatives over physisorbents for CO2 sorption from flue gas.15–17 Lee et al.16,17 synthesized excellent adsorbent with 87–119 mg CO2 per g sorption capacity at 60 °C and 1 bar by impregnation of potassium carbonate in ZrO2, AC, TiO2, Al2O3, MgO, SiO2 and various zeolites. However, regeneration temperature (130–400 °C) and poor cyclic performance of adsorbents became less favourable for CO2 capture. Amines are highly selective towards CO2 via carbamate formation in low partial pressure as well as stable and easily regenerable at moderate temperature even in moist environment.18–29 Aminosilane functionalized OMS is a suitable alternative for CO2 sorption from anthropogenic source.
Since after the discovery of OMS by Mobil scientists in the early 1990s,30 other OMSs like SBA-15 (ref. 22 and 27) (hexagonal), KIT-6 (ref. 28) (cubical), MCF31 (disordered) and MSU32 (hexagonal disordered) were also discovered. Due to high specific surface area (>600 m2 g−1) and pore volume (>0.5 cm3 g−1), they have attracted world-wide researcher's interest in various applications like catalysis,33 nano-science34 and drug delivery35 by decorating the surface with organosilane. Yokoi et al.19 synthesized the functionalized MCM-41 by direct co-condensation reaction with aminosilane and silica source during synthesis as well as by post grafting method. Among these, amine functionalized OMSs received the attention of a large community in CO2 capture application. More recently, Linneen et al.25 grafted mono, di and triaminosilane on silica aerogel with larger pore size (42.7 nm) and pore volume (4.2 cm3 g−1) and studied the CO2 adsorption performance. It was found that, the adsorption capacity was increased with increasing amine chain length and maximum for triaminosilane grafted aerogel (1.64 mol CO2 per kg at 25 °C and 1 bar). Hiyoshi et al.21 improved the aminosilane loading by improving the surface properties of SBA-15 by boiling in water followed by post grafting. Sayari et al.36 reported that adsorption is directly associated to amine content present in the adsorbent. Zeleňák et al.29 showed the three dimensional with inter connected porous structure provides faster response during CO2 uptake than long hexagonal porous structure. We recently demonstrated that aminopropyl grafting in aqueous solution was more significant for aminosilane loading as well as CO2 adsorption over anhydrous grafting.29
The physical properties of the support and method of grafting play an important role in the performance of the adsorbent. In the present study, we focus on in-depth understanding in designing the highly CO2/N2 selective aminosilane grafted adsorbent. In view of exploiting the presence of more amino nitrogen for capturing more CO2, this study attempted selection of the best one from the group of commonly used mesoporous adsorbents. In the process of investigation, the traditional MCM-41, SBA-15 and KIT-6 mesoporous silica were synthesized by liquid crystal templating mechanism. Thereafter, they were functionalized with N1-(3-trimethoxysilylpropyl)diethylenetriamine by grafting method in the anhydrous and aqueous solution. The adsorbents were characterized by a various analytical and spectroscopic techniques and also by CO2/N2 uptake measurements under different conditions. The adsorption capacity was compared to decide upon the best support for CO2 adsorption. The variations in adsorption capacities are also elucidated by grafting mechanism and structural properties of the adsorbents.
2. Experimental
Materials
The chemicals pluronic EO–PO–EO triblock copolymer (P123, Sigma-Aldrich), N1-(3-trimethoxysilylpropyl)diethylenetriamine (TMPTA, Sigma) cetrimide (CTAB, Merck), tetraethyl orthosilicate (TEOS, Sigma-Aldrich), ammonia solution (NH3 25%, Merck), hydrochloric acid (HCl, 35%, Merck), 1-butanol (BuOH, Merck), ethanol (EtOH, Merck), and anhydrous toluene were used without any further treatment.Synthesis of ordered mesoporous silica
MCM-41 was synthesized by following procedure:37 2.0 g CTAB was dissolved in 120 mL Millipore purified water. After complete dissolution, 9.0 mL of NH3 was added. The 10 mL of TEOS was added drop wise in solution and stirred for another 12 h at room temperature. The white solid product was filtered, washed with distilled water and dried at 100 °C for 24 h. Surfactant free MCM-41 was obtained after calcination at 550 °C for 5 h.SBA-15 was synthesized by modified procedure:22 4.0 g of P123 was dissolved in 144 mL of 1.7 N HCl at 40 °C. After it became homogeneous, 8.0 g TEOS was added in the solution and stirred for another 24 h at 40 °C. The resulting mixture was transferred in Teflon lined autoclave and aged at 100 °C for 24 h. The white solid product was obtained by filtration and dried at 100 °C for 24 h. Surfactant free SBA-15 was obtained after calcination at 550 °C for 5 h.
KIT-6 was synthesized as earlier reported procedure.28 In typical synthesis, 4.0 g of P123 was dissolved in a mixture of 144 mL water and 7.9 g HCl at 40 °C. Next, 4.0 g of 1-butanol was added in the solution and mixed for another 1 h. The 8.6 g of TEOS was added in the solution and continuously stirred for 24 h at 40 °C. Resulting slurry was transferred into Teflon lined autoclave and hydrothermally treated at 100 °C for 24 h. The white solid product was obtained after filtration of solution and dried in hot air oven at 100 °C. Surfactant free KIT-6 was obtained after calcination at 550 °C for 5 h.
Synthesis of aminosilane grafted OMSs
OMSs was functionalized with N1-(3-trimethoxysilylpropyl)diethylenetriamine by post grafting method.23 Before grafting, 1.0 g of OMS (MCM-41, SBA-15 and KIT-6) was dried at 120 °C in vacuum to remove the pre adsorbed moisture. In anhydrous grafting, 1.0 g of OMS was dispersed in 150 mL toluene and stirred for 1 h to become homogeneous. Then, ‘x’ mL of TMPTA was added in the solution and refluxed at 85 °C in stirring condition for 24 h. The TMPD grafted OMS was filtered and washed repeatedly with toluene and ethanol. The solid product was dried at 80 °C in high vacuum for 16 h and stored for further analysis. The obtained samples are designated as M‘x’T, S‘x’T and K‘x’T of MCM-41, SBA-15 and KIT-6, respectively of base material. In aqueous grafting, after complete dispersion of OMS in toluene, 0.10 mL Millipore purified water was added and stirred for 3 h to modify the silica surface. The further steps were similar as discussed in dry grafting. The resulting samples are denoted as WM20T, WS20T and WK30T.Material characterization
High resolution X-ray powder diffraction spectra were recorded by Bruker D8 advance diffractometer using CuKα radiation operating at 40 kV and 40 mA. Surface micrographs of synthesized OMSs were recorded by field emission scanning electron microscope (FESEM) (Zeiss, Sigma). Transmission electron micrograph (TEM) was recorded using a Jeol (JEM 2100, 200 keV) instrument. Nitrogen adsorption/desorption isotherm was recorded on Quantachrome automated volumetric gas sorption analyzer (autosorb iQ) at −196 °C. Before analysis, samples were degassed at 120 °C for 3 h in ultra-high vacuum. Specific surface area (SBET) was calculated by Brunauer–Emmett–Teller (BET) method. The pore volume (vt) was calculated by volume adsorbed basis at relative pressure 0.99. Pore size distribution was calculated by Barrett–Joyner–Halenda (dBJH) method. Thermal analysis and aminosilane present in the adsorbents were analyzed by thermogravimetry (TG, Netzsch) analyzer.CO2/N2 adsorption
CO2/N2 adsorption measurements were performed in high pressure volumetric gas adsorption apparatus (iSorbHP1-XKRLSPN100). A sample weight of ∼250 mg was loaded into a sample holder and fitted with instrument. Before analysis, pre-adsorbed moisture and gases were removed by degassing the adsorbent at 110 °C for 3 h in ultra-high vacuum and measured the actual weight of adsorbent. CO2/N2 adsorption measurement was conducted at 30, 45, and 60 °C. The adsorbate gases, carbon dioxide (CO2, 99.999%) and nitrogen (N2, 99.999%) were obtained from Assam air product.3. Results and discussion
Characterisation of the aminosilane grafted OMSs
The small angle X-ray diffraction patterns before and after TMPTA grafted MCM-41, SBA-15 and KIT-6 are shown in Fig. 1. In diffraction spectra, peaks observed at 2.6° (100), 0.90° (100) and 0.93° (211) for MCM-41, SBA-15 and KIT-6 mesoporous silica, receptively clearly indicates the synthesized OMSs are highly ordered in nature with corresponding hexagonal, hexagonal and la3d bicontinuous cubic lattice structure.26–30 Fig. 2 shows the corresponding FESEM and TEM micrograph of pure MCM-41, SBA-15 and KIT-6. As seen in Fig. 2a, MCM-41 exhibits monodispersed thin platelets with average particle size ∼ 0.6 μm.37 Fig. 2b shows that synthesized SBA-15 is a road shaped particles structure in a highly ordered bundle.38 KIT-6 exhibits as a particle with average size ∼ 1 μm as shown in Fig. 2c. TEM micrographs of MCM-41, SBA-15 and KIT-6 clearly show the highly ordered structure of the synthesized material as reported in literature.27–30 After grafting of TMPTA, peak intensity is significantly reduced in MCM-41 and sparsely reduced in SBA-15 and KIT-6 (Fig. 1). This is possibly due to complete pore filling in MCM-41 and partial pore filling in SBA-15 and KIT-6.39 In addition, the peaks (110, 200), (110, 200) and (211) are diminishing after grafting of TMPTA in MCM-41, SBA-15 and KIT-6, respectively (Fig. 1). Thus, with increasing amine concentration in the solution, peak intensity decreased in all the adsorbents. In case of aqueous grafted adsorbent (Fig. 1), intensity of the major peak drastically reduced due to higher aminosilane loading.25,28,36 | ||
| Fig. 1 X-ray diffraction pattern of ordered mesoporous silica before and after TMPTA grafting on (a) MCM-41 (b) SBA-15 and (c) KIT-6 adsorbents. | ||
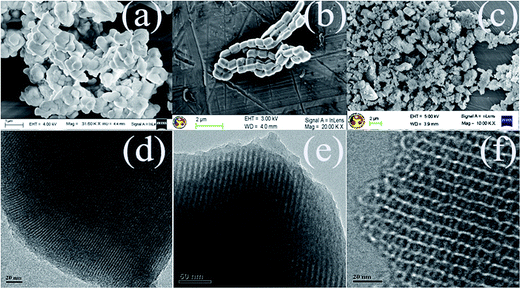 | ||
| Fig. 2 FESEM micrograph of (a) MCM-41 (b) SBA-15, (c) KIT-6 and TEM micrograph of (d) MCM-41 (e) SBA-15 and (f) KIT-6 mesoporous silica. | ||
The nitrogen adsorption/desorption isotherm and pore size distribution of pure and aminosilane grafted OMSs are shown in Fig. 3 and physical properties are summarized in Table 1. All the OMSs demonstrate the type IV isotherm which is general characteristic of mesoporous (2–50 nm) material.22,23,28,30 However, SBA-15 and KIT-6 shows the type H1 hysteresis loop, which is characteristic of material with interconnected large cylindrical pore geometry and high degree of pore size uniformity.40 However, MCM-41 does not show any hysteresis, which is mainly for narrow slit like pores present in the material and also pore size close to micropore range.40 The specific surface area (SBET) of mesoporous MCM-41 (2.2 nm), SBA-15 (6.6 nm) and KIT-6 (6.6 nm) was 1492, 857 and 860 m2 g−1, respectively. After anhydrous aminosilane grafting on MCM-41, SBET and pore volume are sharply reduced whereas SBET of SBA-15 and KIT-6 approximately become 2/5th of the original. This is obviously caused by the aminosilane grafting in the channels and internal mesoporous surface. But, approximately 50% of the pore volume of SBA-15 and KIT-6 remain empty. The surface area and pore volume of MCM-41, SBA-15 and KIT-6 are sharply reduced after aqueous aminosilane grafting (Table 1) and is due to higher amount of aminosilane grafting in presence of water. It may be noticed that, pore diameters of hexagonal MCM-41 and SBA-15 are highly reduced after aqueous aminosilane grafting whereas that of KIT-6 is not much affected. This is because of the presence of inter-connected porous channels in KIT-6.
 | ||
| Fig. 3 Nitrogen adsorption/desorption of before and after aminosilane grafting of (a) MCM-41 (b) SBA-15, (c) KIT-6 at −196 °C and pore size distribution of (d) MCM-41 (e) SBA-15 and KIT-6 adsorbents. | ||
| OMSs | SBET (m2 g−1) | vt (cm3 g−1) | dBJH (nm) |
|---|---|---|---|
| a SBET: specific surface area, vt: pore volume, dBJH: pore diameter. | |||
| MCM-41 | 1492 | 0.91 | 2.2 |
| M15T | 107 | 0.16 | 2.1 |
| M20T | 82 | 0.16 | 1.6 |
| M25T | 77 | 0.16 | 1.6 |
| WM20T | 31 | 0.005 | 1.6 |
| SBA-15 | 857 | 1.23 | 6.6 |
| S15T | 321 | 0.57 | 4.9 |
| S20T | 301 | 0.53 | 4.9 |
| S25T | 281 | 0.50 | 4.9 |
| WS20T | 24 | 0.007 | 1.35 |
| KIT-6 | 860 | 1.24 | 6.6 |
| K20T | 316 | 0.61 | 5.6 |
| K25T | 312 | 0.61 | 5.6 |
| K30T | 310 | 0.59 | 5.6 |
| K35T | 305 | 0.59 | 5.6 |
| WK30T | 190 | 0.36 | 4.9 |
| WK30T | 55 | 0.12 | 4.3 |
FTIR analysis
The mechanism of aminosilane grafting over OMSs and CO2 interaction with functionalized adsorbent is discussed through the IR-spectra as shown in Fig. 4. Surface properties of MCM-41, SBA-15 and KIT-6 before grafting are compared using IR-spectra. In all the OMSs, a peak with a wide shoulder 3600–3400 cm−1 ascribes the presence of hydrogen-bonded silanol group (Si–OH) and 3750 cm−1 for free silanol group present on the surface.41 Other peaks at 1250–1040 cm−1, and ∼799 cm−1 assign the asymmetric and symmetric stretching vibration of Si–O–Si silanol bridge. The peaks at 960 cm−1 represents the Si–O in-plane stretching vibrations of free silanol groups and ∼1620 cm−1 for adsorbed water present on the silica surface.33,42 After TMPTA grafting in OMSs, some new peaks evolved and some peaks disappeared in the spectra. The peak corresponds to 960 cm−1 was completely disappeared in the aminosilane grafted adsorbents because of grafting of aminosilane with the free silanol group present on the surface. The band at 2940–2880 cm−1 and 1410 cm−1 attributed to C–H and C–N stretching of aminosilane, respectively.23,33,43 The peaks at 1630, 1580 and 1309 cm−1 are associated with chemical reaction between CO2 and amine and formation of ammonium ions (NH3+), carbamate (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)− and NCOO− skeletal vibration, respectively.43
C)− and NCOO− skeletal vibration, respectively.43
CO2/N2 adsorption on MCM-41, SBA-15 and KIT-6
The selection of appropriate mesostructured support can substantially reduce the size of the capture equipment and the cost. The CO2 sorption capacity of sorbent is a primary objective in designing the separation process. Thus, in the present case, initially CO2 adsorption was performed on pure OMSs at different temperatures to understand the effect of aminosilane loading. Before adsorption experiment, OMSs were degassed at 150 °C for 3 h in ultra-high vacuum to remove the pre-adsorbed moisture and CO2. The CO2 adsorption isotherms on MCM-41, SBA-15 and KIT-6 at different temperatures 30, 45 and 60 °C under pressure range up to 1 bar are shown in Fig. 5. The equilibrium adsorption capacity (qe) almost linearly increases with increasing the pressure over all the OMSs. The following trend of qe is observed for different OMSs: SBA-15 (0.74 mol CO2 per kg) > KIT-6 (0.64 mol CO2 per kg) > MCM-41 (0.56 mol CO2 per kg) at 30 °C and 1 bar. The lowest qe of MCM-41 even after the highest SBET compared to SBA-15 and KIT-6 is possibly for different surface properties. SBA-15 (3.4–8.5 OH per nm2)22 contains more surface silanol than MCM-41 (2.5–3.0 OH per nm2),41 that resulted in the highest qe. In case of KIT-6, possibly contains intermediated number of silanol groups on the surface. A known fact, the adsorption capacity is strongly influenced by temperature. The amount of CO2 adsorbed on MCM-41 is decreased with increase in temperature and similar trends are observed with SBA-15 and KIT-6 as shown in Fig. 5.38 | ||
| Fig. 5 CO2 adsorption on (a) MCM-41, (b) SBA-15, and (c) KIT-6 and corresponding N2 adsorption in (d), (e) and (f), respectively at 30 °C. | ||
The N2 adsorption isotherm on MCM-41, SBA-15 and KIT-6 under pressure range 1 bar is shown in Fig. 5. The qe is linearly increases with pressure and as following the order MCM-41 (0.04 mol N2 per kg) < SBA-15 (0.04 mol N2 per kg) < KIT-6 (0.05 mol N2 per kg) at 30 °C and 1 bar.
Effect of aminosilane concentration
To understand the effect of aminosilane concentration in the solvent on grafting capacity as well as on qe, a series of experiments were performed by varying the TMPTA to OMSs ratio and results are summarized in Fig. S1.† The hexagonal structured MCM-41 and SBA-15 show maximum CO2 qe for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (TMPTA
1 (TMPTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OMS) ratio. But, in case of three dimensional KIT-6, 3
OMS) ratio. But, in case of three dimensional KIT-6, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (TMPTA
1 (TMPTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OMS) ratio gives maximum adsorption capacity. This qe is reduced marginally with higher concentration. The TMPTA intensity at the internal surface is increased but the active site is reduced due to overlapping with each other.25,28,44 The degree of grafting on OMS is in the following order KIT-6 > SBA-15 > MCM-41. Diffusional resistance and pore blocking can control the movement of amine into the interior of the pore. Presence of interconnected pore in KIT-6 reduces the diffusional resistance and thereby reduces the hindrance of the movement of amine for grafting.29 This in turn reduces the pore blocking also.
OMS) ratio gives maximum adsorption capacity. This qe is reduced marginally with higher concentration. The TMPTA intensity at the internal surface is increased but the active site is reduced due to overlapping with each other.25,28,44 The degree of grafting on OMS is in the following order KIT-6 > SBA-15 > MCM-41. Diffusional resistance and pore blocking can control the movement of amine into the interior of the pore. Presence of interconnected pore in KIT-6 reduces the diffusional resistance and thereby reduces the hindrance of the movement of amine for grafting.29 This in turn reduces the pore blocking also.
Extracted information from anhydrous grafting is used further for aqueous grafting as the latter is a more efficient way to graft the aminosilane in OMSs. In the present study, water to OMSs ratio (0.1) in solution were maintained during grafting of aminosilane. The qe of aqueous grafted adsorbent (WM20T and WS20T) was reduced to 0.66 and 0.64 mol CO2 per kg from 1.36 (M20T) and 1.54 (S20T) mol CO2 per kg of adsorbent as shown in Fig. 6. Whereas, the qe of WK30T were increased from 1.89 mol CO2 per kg to 2.59 mol CO2 per kg at 30 °C and 1 bar. This suggests that the surface architecture and mesopore size of OMS affects the degree of silylation and qe. However, aminosilane loading was higher during aqueous grafting than anhydrous as explained in the section below.25,36 The reduction in qe of aqueous grafted adsorbents corresponds WM20T and WS20T is possibly due to plugging the pores of MCM-41 and SBA-15 through inter aminosilane condensation reaction.
Effect of temperature on CO2/N2 adsorption
Sorption temperature as well as capacity of adsorbent are essential parameters in designing the adsorption process. In the present study, CO2/N2 adsorption isotherm on M20T, S20T and K30T at (30, 45, and 60) °C in the pressure range of 0–1.0 bar were performed and the results are depicted in Fig. 7. The CO2 qe of all the aminosilane grafted adsorbents progressively decreases with increasing the temperature. The maximum qe is obtained at 30 °C as per the following order K30T (1.89 mol CO2 per kg) > S20T (1.54 mol CO2 per kg) > M20T (1.36 mol CO2 per kg). When the temperature is increased to 60 °C, qe becomes 1.63, 1.04, 1.01 mol CO2 per kg of K30T, S20T and M20T respectively at 1 bar. | ||
| Fig. 7 CO2 adsorption isotherms for (a) M20T (b) S20T and (c) K30T and N2 adsorption isotherm in (d) M20T, (e) S20T and (f) K30T at different temperatures (■: 30 °C) (●: 45 °C) and (▲: 60 °C). | ||
The similar trends of CO2 adsorption isotherm over aqueous grafted adsorbent (WK30T) were observed at different temperatures. However, reduction in CO2 adsorption capacity of WK30T with temperature is much less as shown in Fig. 8. The qe is 2.59, 2.47 and 2.38 mol CO2 per kg at 30, 45 and 60 °C, respectively. The values are much higher than M20T and S20T. Table 2, summarized the maximum qe and compared with other TMPTA grafted mesoporous silica. As can be seen from the table, WK30T is better performing with respect to other adsorbents. It is further observed that, larger pore mesoporous silica gives better qe after TMPTA aqueous grafting compared to smaller size mesoporous silica.45–48
 | ||
| Fig. 8 (a) CO2 and (b) N2 adsorption isotherms on WK30T at different temperatures (■: 30 °C) (●: 45 °C) and (▲: 60 °C). | ||
| OMSs | dp (nm) | Adsorption condition (°C/bar) | Adsorption capacity (mol CO2 per kg) | Methods | Reference |
|---|---|---|---|---|---|
| a Aqueous grafting. | |||||
| MCM-41 | 2.7 | 30/1 | 1.01 | Manometric gas dosing system | 26 |
| PE-MCM-41 | 11.7 | 25/1 | 2.50a | Gravimetric | 45 |
| SBA-15 | 8.9 | 45/1 | 1.8 | Gravimetric | 46 |
| HMS | 2.21 | 20/1 | 1.1 | TGA/DTA | 47 |
| Silica aerogels | 42.7 | 25/1 | 2.61a | Electro-microbalance | 25 |
| SBA-15-p | 14.7 | 25/1 | 2.67 | Volumetric | 48 |
| SBA-15-f | 7.5 | 25/1 | 1.23 | Volumetric | 48 |
| MCM-41 (M20T) | 2.2 | 30/1 | 1.36 | Volumetric | Present study |
| SBA-15 (S20T) | 6.6 | 30/1 | 1.54 | Volumetric | Present study |
| KIT-6 (K30T) | 6.6 | 30/1 | 1.89 | Volumetric | Present study |
| KIT-6 (WK30T) | 6.6 | 30/1 | 2.59a | Volumetric | Present study |
Multi temperature dual-site Langmuir (DSL) model6,12 explained in the ESI† is used to describe the CO2 adsorption behaviour on TMPTA grafted adsorbents at different temperatures. The DSL model (continuous line) (Fig. 7 and 8) show excellent agreement with the experimental data (solid points) at different temperatures.
The reduction in CO2 adsorption capacity over amine grafted adsorbents can be explained by the classical adsorption mechanism. The CO2 adsorption on amine grafted OMSs is an exothermic process and it becomes more favourable at lower temperature. Compared with some other aminosilane functionalized adsorbent, similar behaviour was observed in the present study.23,27,48 Whereas, Sayari et al.49 showed the qe of TMPTA grafted PE-MCM-41 was somewhat increased with increasing the temperature. It facilitates the diffusion of CO2 in aminosilane layer with temperature and increased the active amine sites for interaction. The similar adsorption behaviour was also observed in polyamine impregnated mesoporous silica.31,32,39 But in the present case, CO2 diffusion in aminosilane layer with temperature is dominated by the desorption process and reduced the capacity at higher temperature.
During grafting, aminosilane cover a fraction of the surface.46 Hereafter, sorption capacity of amine functionalized adsorbent is combined effect of chemical and physical adsorption.49 In a low CO2 partial pressure (∼till 0.15 bar), the sorption capacity of M20T, S20T and K30T increased sharply and it was mainly for chemical adsorption between CO2 and grafted aminosilane.32,39 Moreover, at higher CO2 partial pressure sorption capacity gradually increases with pressure and it was probably for physical adsorption with the surface.49
Low qe of N2 indirectly indicates the high CO2/N2 selectivity of the adsorbent. Fig. 7d–f show the effect of temperature (30, 45 and 60 °C) on N2 adsorption performance over M20T, S20T and K30T. Nitrogen qe at 30 °C and 1 bar was 0.019, 0.012 and 0.011 mol N2 per kg of M20T, S20T and K30T adsorbent, respectively. There was no major difference in qe of all the adsorbents and moreover not much affected with adsorption temperature. In case of aqueous grafted WK30T, qe was 0.013, 0.008 and 0.007 mol N2 per kg adsorbent at 30, 45 and 60 °C at 1 bar, respectively as shown in Fig. 8b. The adsorption capacity of N2 in amine grafted mesoporous silica is much lower than other adsorbents like MOF and polymeric adsorbent,12,14,50 which makes amine functionalized mesoporous silica a lucrative option over other adsorbents. Additionally, the qe of CO2 is much higher than that of N2 at all temperatures in all the adsorbents because of the greater quadrupole moment and polarizability of CO2 (4.30 × 10−26 esu cm2 and 29.11 × 10−25 cm3, respectively) than N2 (1.52 × 10−26 esu cm2 and 17.403 × 10−25 cm3, respectively).51
The important parameter before selection of any adsorbent is the selectivity. It is defined by Sx/y = (qei/qej)/(pi/pj); where qej, qej and pi, pj are the amount adsorbed and corresponding partial pressure for component i and j, respectively.52 The CO2 and N2 (∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) is a major component of flue gas emitted from coal based thermal power plant. In the present study, on the basis of this composition the selectivities at 30 °C are 14, 23 and 15 of MCM-41, SBA-15 and KIT-6, respectively. After TMPTA grafting, selectivity is improved to 491, 635, 793 and 873 of M20T, S20T, K30T and WK30T, respectively.
85) is a major component of flue gas emitted from coal based thermal power plant. In the present study, on the basis of this composition the selectivities at 30 °C are 14, 23 and 15 of MCM-41, SBA-15 and KIT-6, respectively. After TMPTA grafting, selectivity is improved to 491, 635, 793 and 873 of M20T, S20T, K30T and WK30T, respectively.
Enthalpy of adsorption
Enthalpy of adsorption (ΔEads) is calculated by Clausius–Clapeyron relation.12 The −ΔEads values for CO2 on pure MCM-41, SBA-15 and KIT-6 are ca. 18, 20 and 18 kJ mol−1 and constant with loading.23,26 At zero coverage of CO2 adsorption (Fig. 9) over TMPTA grafted adsorbents; −ΔEads value is much higher than the pure OMSs.22,26 This is due to the chemical interactions between CO2 and amine present on the surface as earlier confirmed by several authors.22–24,49 The −ΔEads values are summarized in the Table 3. A sharp reduction in enthalpy is observed with increase in loading at higher pressure. It confirms fact that the amine functionalized silica contains two different types of site for CO2 adsorption as earlier explained by Sayari et al.45 which is namely amine and bare silica surface. In the initial phase higher enthalpy value is for major contribution from chemisorption with amine and later lower for major contribution from physical interaction with the surface because of exhaustion of amine.| OMSs | qe (mol CO2 per kg) | (mol N per kg) | % amine efficiency mol(CO2)/mol(N) | Selectivity at 30 °C | ΔEads (kJ mol−1) | |
|---|---|---|---|---|---|---|
| 30 °C | 60 °C | |||||
a qe: equilibrium adsorption capacity at 1 bar; selectivity: calculated at 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85 partial pressure ratio, ΔEads: enthalpy of adsorption. 0.85 partial pressure ratio, ΔEads: enthalpy of adsorption. |
||||||
| MCM-41 | 0.56 | 0.39 | — | — | 14 | 18 |
| SBA-15 | 0.74 | 0.48 | — | — | 23 | 20 |
| KIT-6 | 0.64 | 0.40 | — | — | 15 | 18 |
| M20T | 1.36 | 1.14 | 3.72 | 36.6 | 491 | 31 |
| WM20T | 0.67 | — | 4.70 | 14.2 | — | — |
| S20T | 1.54 | 1.18 | 3.98 | 38.7 | 635 | 67 |
| WS20T | 0.63 | — | 5.46 | 8.7 | — | — |
| K30T | 1.89 | 1.73 | 4.25 | 44.8 | 793 | 42 |
| WK30T | 2.59 | 2.38 | 5.73 | 45.1 | 873 | 33 |
TGA analysis
The amount of TMPTA present in the adsorbents was analyzed by TG analysis.24,42 Fig. S2 (ESI†) shows the TG curves of aminosilane grafted MCM-41, SBA-15 and KIT-6 and the corresponding differential thermal gravimetric (DTG) curves are shown in Fig. 10. TG curves clearly show that the organic aminosilane completely degrade within 750 °C. A sharp weight loss in all the adsorbents is observed in the initial temperature range 30–150 °C, which is attributed to the loss of moisture and adsorbed gases. The three peaks observed in Fig. 10 in the ranges (150–250), (250–500) and (500–750) °C are due to decomposition of grafted aminosilane.24,36,42 The corresponding amine group numbers (mol N per kg-adsorbent) are summarized in Table 3. The amine groups available for CO2 during adsorption is the highest in KIT-6 and the least in MCM-41 for both dry and anhydrous grafted adsorbents. During grafting in MCM-41 and SBA-15 with cylindrical pore mouth gets easily plugged by TMPTA. But in KIT-6, interconnected channel provides better surface accessibility for grafting of guest molecule and accommodate higher amount of amine. The similar phenomenon was also reported by Zeleňák et al.29 with aminopropyl grafting over different porous structures. | ||
| Fig. 10 Differential thermal gravimetric (DTG) analysis of pure and TMPTA grafted (a) MCM-41 (b) SBA-15 and (c) KIT-6 mesoporous silica. | ||
Amine efficiency is the ratio of CO2 adsorbed (mol CO2 per kg) to amine group number (mol N per kg) present in the adsorbent.27,47,48 Hence, theoretical maximum possible amine efficiency is 0.5. TMPTA consists of one primary amine and two secondary amines. Primary and secondary amine of TMPTA reacts with CO2 and formed carbamate with zwitterion intermediate,27 where two amines reacts with one molecule of CO2 under dry condition. Efficiency of grafted aminosilane in CO2 adsorption increases with increase in the pore diameter of OMSs and it follows the order K30T > S20T > M20T. In case of hexagonal aqueous grafted adsorbents (WM20T and WS20T), efficiency decreased sharply even after higher amine loading. This indicates that the hexagonal pore has a diffusional limitation for CO2 adsorption possibly due to pore blocking by condensation reactions between aminosilanes during grafting (Scheme 1). The similar trends were observed by several authors in recent study over amine functionalized adsorbent.39,44 In case with WK30T, amine group number and CO2 adsorption capacity proportionally increased. It is due to the mesoporous inter connected channels present in KIT-6 thus, reducing the said limitation.
Grafting mechanism
The main prerequisite in developing an efficient amine functionalized adsorbent is the selection of base mesoporous silica. In the present study, we grafted the aminosilane on high surface area OMSs with different structure (hexagonal, cubical), pore size and pore volume. The qe of amine functionalized adsorbent is directly associated with assessable amine groups for CO2 during adsorption.22 A schematic grafting procedure is shown in Scheme 1. Fig. 3 shows the synthesized support MCM-41 (2.2 nm), SBA-15 (6.6 nm) and KIT-6 (6.6 nm) have different pore sizes. All the mesoporous silica enriches with surface silanol groups and participates in grafting reaction. In anhydrous grafting, aminosilane directly reacts with surface silanol groups by condensation reaction (Scheme 1b) and grafted over the surface. However, lower adsorption capacity of M20T is possibly for lower amine loading for smaller pore size of MCM-41 as reflected from Table 1.TMPTA is highly sensitive to moisture. In the presence of water, TMPTA forms a cluster via hydrolysis reaction.53 However, water also gets adsorbed on the negatively charged siloxane bridge of mesoporous silica and raptures the surface.54 In this process, it creates new surface silanol groups on the surface, which also promote the aminosilane grafting capacity. The qe of the aqueous grafted adsorbents are summarized in Table 2 and the grafting mechanism is shown in Scheme 1c. The higher accumulation of aminosilane in WM20T, WS20T and WK10T is the combined effect of increment in surface silanol groups and hydrolysis reaction between aminosilanes. During grafting, aminosilane forms a cluster in internal surface of the adsorbents.53 The qe of WM20T and WS20T was reduced, however increased in WK10T, even though after, the aminosilane loading was increased in all the adsorbents as analyzed by TG analysis. The increments in aminosilane loading are also understood by reduction in surface area and pore volume (Table 1).
The structure and pore size of the support plays an important role in the performance of adsorbent. The reduction in adsorption capacity can be correlated with the support structure and pore opening. During aqueous aminosilane grafting, pore of the hexagonal mesoporous silica MCM-41 and SBA-15 was blocked by aminosilane condensation reaction as shown in Scheme 1c and become a nonporous adsorbent as was also observed by N2 adsorption/desorption analysis (pore volume becomes zero). The approximately same qe of WM20T (0.64 mol CO2 per kg) and WS20T (0.62 mol CO2 per kg) corroborates the above statement. During grafting in KIT-6, TMPTA forms a cluster in the channels by condensation and enhances the aminosilane loading. But it does not block the channels during grafting and enhanced CO2 adsorption capacity by increasing the active amine sites. The enhancement in qe of WK10T is the consequence of the above fact.
Reusability performance of adsorbents
In order to have practical applicability, stable sorption capacity in cyclic use is a primary criterion of a good adsorbent. In the present study, 20 adsorption/desorption cycles were subjected over M20T, S20T, K30T and WK30T at 30 °C. After each cycle of adsorption, samples were degassed at 95 °C for an hour. All the adsorbents showed a stable sorption capacity till 20 cycles as shown in Fig. 11. This indicates the stable attachment of aminosilane with the silica surface and no amine loss to the environment.254. Conclusion
A series of ordered mesoporous silica samples namely MCM-41, SBA-15 and KIT-6 were synthesized by liquid crystal template mechanism and functionalized with TMPTA in anhydrous and aqueous amine solution. The synthesized adsorbents were subjected to CO2/N2 adsorption at various conditions. The adsorption capacity of anhydrous grafted adsorbents was followed the order as K30T (1.89 mol CO2 per kg) > S20M (1.54 mol CO2 per kg) > M20T (1.36 mol CO2 per kg) at 30 °C and 1 bar. Moreover, adsorption capacity was increased to 2.59 mol CO2 per g after aqueous grafting over KIT-6. The CO2/N2 selectivity of the KIT-6 based adsorbent is much higher than MCM-41 and SBA-15. The lower adsorption capacity as well as selectivity of TMPTA grafted MCM-41 and SBA-15 makes them less favourable in particle application. In addition to CO2 adsorption, WK30T is easily regenerable at moderate temperature and showed good stability for several adsorption/adsorption cycles. Intermolecular condensation of aminosilane blocks the pores and thereby reduces the CO2 sorption capacity of TMPTA grafted MCM-41 and SBA-15. However, presence of interconnected channels in KIT-6 eliminates that possibility and made the TMPTA grafted KIT-6 with high CO2 adsorption capacity and excellent CO2/N2 selectivity a potential adsorbent for commercial application.Acknowledgements
The authors would like to acknowledge Central Instrument Facility (CIF) of Indian Institute of technology Guwahati (IITG) for Nitrogen adsorption/desorption analysis at −196 °C, CO2/N2 adsorption analysis, FESEM and TEM analysis.References
- Annual energy outlook 2015 with projections to 2040. (http://www.eia.gov/forecasts/aeo/).
- F. M. Orr Jr, CO2 capture and storage: are we ready?, Energy Environ. Sci., 2009, 2, 449–458 Search PubMed.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreibera and T. E. Müller, Energy Environ. Sci., 2012, 5, 7281–7305 CAS.
- R. Idem, M. Wilson, P. Tontiwachwuthikul, A. Chakma, A. Veawab, A. Aroonwilas and D. Gelowitz, Ind. Eng. Chem. Res., 2006, 45, 2414–2420 CrossRef CAS.
- O. Cheung and N. Hedin, RSC Adv., 2014, 4, 14480–14494 RSC.
- S. García, J. J. Pis, F. Rubiera and C. Pevida, Langmuir, 2013, 29, 6042–6052 CrossRef PubMed.
- D. Lin, X. Zhang, X. Cui and W. Chen, RSC Adv., 2014, 4, 27414–27421 RSC.
- J. A. Mason, T. M. McDonald, T.-H. Bae, J. E. Bachman, K. Sumida, J. J. Dutton, S. S. Kaye and J. R. Long, J. Am. Chem. Soc., 2015, 137, 4787–4803 CrossRef CAS PubMed.
- S. Xiang, Y. He, Z. Zhang, H. Wu, W. Zhou, R. Krishna and B. Chen, Nat. Commun., 2012, 3, 954 CrossRef PubMed.
- J. Peng, S. Xian, J. Xiao, Y. Huang, Q. Xia, H. Wang and Z. Li, Chem. Eng. J., 2015, 270, 282–289 CrossRef CAS.
- Z. Zhang, Z.-Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868–2899 CAS.
- P. Mishra, S. Edubilli, B. Mandal and S. Gumma, J. Phys. Chem. C, 2014, 118, 6847–6855 CAS.
- H. A. Patel, F. Karadas, A. Canlier, J. Park, E. Deniz, Y. Jung, M. Atilhan and C. T. Yavuz, J. Mater. Chem., 2012, 22, 8431–8437 RSC.
- J. Wang, J. Huang, X. Wu, B. Yuan, Y. Sun, Z. Zeng and S. Deng, Chem. Eng. J., 2014, 256, 390–397 CrossRef CAS.
- F. Karadas, C. T. Yavuz, S. Zulfiqar, S. Aparicio, G. D. Stucky and M. Atilhan, Langmuir, 2011, 27, 10642–10647 CrossRef CAS PubMed.
- S. C. Lee, B. Y. Choi, T. J. Lee, C. K. Ryu, Y. S. Ahn and J. C. Kim, Catal. Today, 2006, 111, 385–390 CrossRef CAS.
- S. C. Lee, H. J. Chae, S. J. Lee, Y. H. Park, C. K. Ryu, C. K. Yi and J. C. Kim, J. Mol. Catal. B: Enzym., 2009, 56, 179–184 CrossRef CAS.
- A. Zhao, A. K. Samanta, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2013, 52, 6480–6491 CrossRef CAS.
- T. Yokoi, H. Yoshitake and T. Tatsumi, J. Mater. Chem., 2004, 14, 951–957 RSC.
- N. Mittal, A. K. Samanta, P. Sarkar and R. Gupta, Energy Sci. Eng., 2015, 3, 207–220 CrossRef CAS.
- N. Hiyoshi, K. Yogo and T. Yashima, Microporous Mesoporous Mater., 2005, 84, 357–365 CrossRef CAS.
- L. Wang and R. T. Yang, J. Phys. Chem. C, 2011, 115, 21264–21272 CAS.
- M. R. Mello, D. Phanon, G. Q. Silveira, P. L. Llewellyn and C. M. Ronconi, Microporous Mesoporous Mater., 2011, 143, 174–179 CrossRef CAS.
- M. Gil, I. Tiscornia, Ó. de la Iglesia, R. Mallada and J. Santamaría, Chem. Eng. J., 2011, 175, 291–297 CrossRef CAS.
- N. N. Linneen, R. Pfeffer and Y. S. Lin, Chem. Eng. J., 2014, 254, 190–197 CrossRef CAS.
- T. C. Santos, S. Bourrelly, P. L. Llewellyn, J. W. M. Carneiro and C. M. Ronconi, Phys. Chem. Chem. Phys., 2015, 17, 11095–11102 RSC.
- Y. G. Ko, S. S. Shin and U. S. Choi, J. Colloid Interface Sci., 2011, 361, 594–602 CrossRef CAS PubMed.
- R. Kishor and A. K. Ghoshal, Chem. Eng. J., 2015, 262, 882–890 CrossRef CAS.
- V. Zeleňák, M. Badaničová, D. Halamová, J. Čejka, A. Zukal, N. Murafa and G. Goerigk, Chem. Eng. J., 2008, 144, 336–342 CrossRef.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T.-W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- J. Zhao, F. Simeon, Y. Wang, G. Luo and T. A. Hatton, RSC Adv., 2012, 2, 6509–6519 RSC.
- D. S. Dao, H. Yamada and K. Yogo, Ind. Eng. Chem. Res., 2013, 52, 13810–13817 CrossRef CAS.
- C. K. P. Neeli, S. Ganji, V. S. P. Ganjala, S. R. R. Kamaraju and D. R. Burri, RSC Adv., 2014, 4, 14128–14135 RSC.
- J. Feng, Z. Wang, B. Shen, L. Zhang, X. Yanga and N. He, RSC Adv., 2014, 4, 28683–28690 RSC.
- M. Vallet-Regi, A. Rámila, R. P. d. Real and J. Pérez-Pariente, Chem. Mater., 2001, 13, 308–311 CrossRef CAS.
- P. J. E. Harlick and A. Sayari, Ind. Eng. Chem. Res., 2007, 46, 446–458 CrossRef CAS.
- S. Loganathan, M. Tikmani and A. K. Ghoshal, Langmuir, 2013, 29, 3491–3499 CrossRef CAS PubMed.
- X. Yan, S. Komarneni and Z. Yan, J. Colloid Interface Sci., 2013, 390, 217–224 CrossRef CAS PubMed.
- W.-J. Son, J.-S. Choi and W.-S. Ahn, Microporous Mesoporous Mater., 2008, 113, 31–40 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- X. S. Zhao, G. Q. Lu, A. K. Whittaker, G. J. Millar and H. Y. Zhu, J. Phys. Chem. B, 1997, 101, 6525–6531 CrossRef CAS.
- V. Zelenak, D. Halamova, L. Gaberova, E. Bloch and P. Llewellyn, Microporous Mesoporous Mater., 2008, 116, 358–364 CrossRef CAS.
- C. S. Srikanth and S. S. C. Chuang, J. Phys. Chem. C, 2013, 117, 9196–9205 CAS.
- Q. Wu, S. Chen and H. Liub, RSC Adv., 2014, 4, 27176–27183 RSC.
- Y. Belmabkhout, G. D. Weireld and A. Sayari, Langmuir, 2009, 25, 13275–13278 CrossRef CAS PubMed.
- R. Sanz, G. Calleja, A. Arencibia and E. S. Sanz-Pérez, Microporous Mesoporous Mater., 2012, 158, 309–317 CrossRef CAS.
- G. P. Knowles, S. W. Delaney and A. L. Chaffee, Ind. Eng. Chem. Res., 2006, 45, 2626–2633 CrossRef CAS.
- L. Zhou, J. Fan, G. Cui, X. Shang, Q. Tang, J. Wang and M. Fan, Green Chem., 2014, 16, 4009–4016 RSC.
- Y. Belmabkhout and A. Sayari, Energy Fuels, 2010, 24, 5273–5280 CrossRef CAS.
- M. G. Rabbani, T. E. Reich, R. M. Kassab, K. T. Jackson and H. M. El-Kaderi, Chem. Commun., 2012, 48, 1141–1143 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J.-X. Hu, H. Shang, J.-G. Wang, L. Luo, Q. Xiao, Y.-J. Zhong and W.-D. Zhu, Ind. Eng. Chem. Res., 2014, 53, 11828–11837 CrossRef CAS.
- C.-H. Chiang, N.-I. Liu and J. L. Koenig, J. Colloid Interface Sci., 1982, 86, 26–34 CrossRef CAS.
- T. A. Michalske and S. W. Freiman, Nature, 1982, 295, 511–512 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The effect of TMPTA concentration (Fig. S1) and TG analysis (Fig. S2). See DOI: 10.1039/c5ra20489e |
| This journal is © The Royal Society of Chemistry 2016 |