In situ preparation and characterization of a conductive and magnetic nanocomposite of polypyrrole and copper hydroxychloride
Subramanyam Kasisomayajula*,
Niteen Jadhav and
Victoria Johnston Gelling
Department of Coatings and Polymeric Materials, North Dakota State University, Fargo, ND 58102, USA. E-mail: kasi@autonomicmaterials.com; Tel: +1 701 306 5156
First published on 3rd December 2015
Abstract
A nanocomposite of conductive polypyrrole and ferrimagnetic copper hydroxychloride (Cu2Cl(OH)3) was prepared in a single-step via in situ chemical oxidation of pyrrole using CuCl2 as an oxidizing agent. In this study, it was shown that by monitoring the reaction time and conditions, the physical and chemical properties of polypyrrole and Cu2Cl(OH)3 in the nanocomposite can be easily controlled. This resulted in a nanocomposite with optimized conductivity and magnetic properties for a wide variety of applications such as magnetic recording, electromagnetic shielding, sensors, and spintronic devices. The obtained conductivity (0.0006–33 S cm−1) and magnetization (10.25–53.39 emu mol−1) measurements of the nanocomposite were within the range suitable for these applications and were achieved by controlling the reaction conditions and thus the composition of the nanocomposite. With FTIR, XPS and UV-Vis spectroscopy, it was observed that as the reaction proceeds with time under controlled conditions, the oxidation of pyrrole in the presence of CuCl2 leads to significant structural changes in polypyrrole as well as gradual precipitation of Cu2Cl(OH)3. XRD and SEM analysis showed the effect of reaction conditions on the crystallinity and the morphology of the polypyrrole/Cu2Cl(OH)3 nanocomposite. While the chemical and structural variations in polypyrrole were correlated with the conductivity of the nanocomposite, measured via the conductive-AFM technique, the changes in magnetic properties of the nanocomposite were mainly attributed to the variations observed in the crystallinity of Cu2Cl(OH)3.
1. Introduction
Conducting polymer nanocomposites, containing magnetic inorganic materials, have recently attracted considerable attention due to their combined electrical, optical and magnetic properties.1–3 They offer potential applications for spintronics, memory devices, sensors, microwave absorption devices, and electromagnetic shielding.4–8 Among these conducting polymer nanocomposites, polypyrrole nanocomposites with various magnetic inorganic materials, have been found to have better prospects for the aforementioned applications because of their good environmental stability, adequate conductivity and excellent redox properties.9,10 Several successful attempts have been made in synthesizing these composites with the combination of enhanced magnetic and conducting properties.11–14 Most of these reports have demonstrated the synthesis and properties of these composites, wherein magnetic inorganic particles, such as FexOy, Fe2O3, γ-Fe2O3 and SiO2, were coated with conducting polymers via chemical or electrochemical oxidation methods. The presence of the nanoparticles of these magnetic inorganic materials in the composite was demonstrated by the characterizing new peaks of the magnetic material in FTIR.15,16 For example, the interaction between polypyrrole and Fe2O3 nanoparticles was investigated by studying the chemical structure of polypyrrole in FTIR and the microstructural differences in the polypyrrole/Fe2O3 nanocomposite in SEM. In contrast, compared to the commonly observed network structure of pure polypyrrole, the microstructures of nanocomposites with different loadings of Fe2O3 exhibited distinctly separated nanoparticles of polypyrrole/Fe2O3.15 However, some of the inorganic materials such as Fe3O4 are very susceptible to oxidation, owing to their high chemical activity.17 Moreover, during functionalization of nanoparticles with conducting polymers, these nanoparticles without any surface modifications are susceptible to aggregation due to their large surface area to volume ratio resulting in poor dispersibility.18Another challenge in the preparation of conductive and magnetic nanocomposites, via incorporation of magnetic nanoparticles into a conductive polymer matrix by either chemical oxidation or electrochemical methods, is the dissolution of magnetic nanoparticles in the acidic solution.15 In the work of Guo et al., longer reaction times caused particle loss due to dissolution during the nanocomposite preparation. As a result, the nanocomposite prepared via this method did not exhibit any magnetic hysteresis. In addition, the dissolution of magnetic nanoparticles also caused inhibition to the polymerization of pyrrole leading to lower yields of polypyrrole.15
Enhancement of the magnetic properties of composites can be attributed to not only a proper dispersion of the magnetic nanoparticles but also to their crystallinity and morphology.19 Sunderland et al. demonstrated that the magnetic properties of nanocomposites are closely associated to the crystallinity of inorganic component in the nanocomposites.20 γ-Fe2O3 nanoparticles synthesized via the emulsion method at room temperature and higher temperatures were used to prepare γ-Fe2O3/polypyrrole nanocomposites. Higher temperatures produced nanoparticles with higher crystallinity. Nanocomposites containing these nanoparticles exhibited a significant improvement in magnetization, while retaining its conductivity.20
The crystalline and magnetic properties of Cu2Cl(OH)3 (atacamite or clinoatacamite), a naturally occurring copper mineral, have been widely studied because of their ferrimagnetic behavior. This mineral is found to exhibit the highest coercive field in copper compounds.21,22 To the best of our knowledge, there is no report on in situ synthesis of a polypyrrole/Cu2Cl(OH)3 nanocomposite via mechanistic studies. In our present study, we attempted to synthesize a polypyrrole/Cu2Cl(OH)3 nanocomposite in a single-step via oxidative reaction of pyrrole in the presence of CuCl2 under controlled conditions. The mechanistic study of the reaction provided the information, including the formation rates of polypyrrole and Cu2Cl(OH)3, as well as the required conditions to control the formation rates. In addition, conductivity measurements showed that the conductive properties of the resultant nanocomposite predominantly depend on the quality and quantity of polypyrrole incorporated into the nanocomposite. Similarly, the magnetization tests confirmed that the magnetic properties of the resultant nanocomposite mainly rely on the characteristics of Cu2Cl(OH)3 deposited in the nanocomposite. The characteristics of both polypyrrole and Cu2Cl(OH)3 in the nanocomposite were analyzed using various techniques such as Fourier transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (XRD), elemental analysis (EA) and scanning electron microscopy (SEM) in order to understand the effect of morphological and structural properties of both polypyrrole and Cu2Cl(OH)3 on the conductive and magnetic properties of the resultant nanocomposite.
2. Experimental
2.1. Synthesis of the polypyrrole/Cu2Cl(OH)3 nanocomposite
Pyrrole purchased from Sigma Aldrich was vacuum distilled and stored in a refrigerator at around 0 °C prior to use. CuCl2 was also purchased from Sigma Aldrich. For synthesis, aqueous solutions of pyrrole and CuCl2 of equal molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were slowly and simultaneously added, while stirring, into a closed round bottom flask at room temperature. Six different reactions were performed by varying the reaction time (t) from 1 to 6 h. Depending on the polymerization rate of pyrrole and the formation rate of Cu2Cl(OH)3, the product of each reaction may contain varying percentages of polypyrrole and Cu2Cl(OH)3. Therefore, to analyze the composition, as well as the properties, the final products of these reactions were collected from precipitate after completion of each reaction time, washed with Millipore water, dried in an oven at 60 °C for 1 h, and labeled as R1, R2, R3, R4, R5 and R6.
1) were slowly and simultaneously added, while stirring, into a closed round bottom flask at room temperature. Six different reactions were performed by varying the reaction time (t) from 1 to 6 h. Depending on the polymerization rate of pyrrole and the formation rate of Cu2Cl(OH)3, the product of each reaction may contain varying percentages of polypyrrole and Cu2Cl(OH)3. Therefore, to analyze the composition, as well as the properties, the final products of these reactions were collected from precipitate after completion of each reaction time, washed with Millipore water, dried in an oven at 60 °C for 1 h, and labeled as R1, R2, R3, R4, R5 and R6.
2.2. Characterization
An aliquot from each reaction solution was taken, and pyrrole, oligomers, Cu2+ and Cu+ were separated using a hexane/water mixture into two separate solutions of hexane containing pyrrole and oligomers, and water containing Cu2+ and Cu+. For measurement of the pyrrole concentration, the maximum absorption of pyrrole, which occurs between 210 and 220 nm, was observed. In the case of measurement of Cu+ and Cu2+, the bicinchoninic acid (BCA) method, which is normally used for protein analysis, was used to make a complex specifically with Cu+. Using this method, the maximum absorption of the Cu+–BCA complex, which gives a strong purple color at 560 nm, was measured to obtain the concentrations of both Cu+ and Cu2+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), accelerating voltage (15 kV) and the scale (1 μm) were specified on each micrograph. For elemental analysis, a LECO CHNS-932 elemental analyzer was used to determine the amounts of carbon, hydrogen and nitrogen present in a sample of approximately 1 mg. The XPS measurements were performed on an SSX-100 system (Surface Science Instruments), equipped with a monochromated Al Kα X-ray source, a hemi-spherical sector analyzer (HSA) and a resistive anode detector. The samples were mounted on a sample stage using adhesive Al tapes on top of double-side carbon tapes. The base pressure of the XPS system was 2.0 × 10−10 Torr. During the data collection, the pressure was 4.0 × 10−9 Torr. The X-ray spot size was 1 × 1 mm2, which corresponded to an X-ray power of 200 W. The survey spectra were obtained with 15–25 scans at 150 eV using 1 eV per step. Powder X-ray diffraction (XRD) data were collected in Bragg–Brentano geometry using a Rigaku Ultima IV multipurpose XRD instrument of Rigaku. A Cu Kα X-ray radiation was used running at 40 kV with current at 44 mA. Phase identification was achieved with the help of software, JADE 9.0.
000), accelerating voltage (15 kV) and the scale (1 μm) were specified on each micrograph. For elemental analysis, a LECO CHNS-932 elemental analyzer was used to determine the amounts of carbon, hydrogen and nitrogen present in a sample of approximately 1 mg. The XPS measurements were performed on an SSX-100 system (Surface Science Instruments), equipped with a monochromated Al Kα X-ray source, a hemi-spherical sector analyzer (HSA) and a resistive anode detector. The samples were mounted on a sample stage using adhesive Al tapes on top of double-side carbon tapes. The base pressure of the XPS system was 2.0 × 10−10 Torr. During the data collection, the pressure was 4.0 × 10−9 Torr. The X-ray spot size was 1 × 1 mm2, which corresponded to an X-ray power of 200 W. The survey spectra were obtained with 15–25 scans at 150 eV using 1 eV per step. Powder X-ray diffraction (XRD) data were collected in Bragg–Brentano geometry using a Rigaku Ultima IV multipurpose XRD instrument of Rigaku. A Cu Kα X-ray radiation was used running at 40 kV with current at 44 mA. Phase identification was achieved with the help of software, JADE 9.0.A Veeco Dimension 3100 atomic force microscope with contact mode and current sensing probe was used for C-AFM to characterize the coatings prepared on aluminum substrate for surface morphology and current density. The platinum–iridium (Pt/Ir) coated cantilevers (Model: SCM-PIC, 0.01–0.025 ohm cm antimony (n) doped Si, spring constant 0.25 N m−1) were purchased from Veeco Instruments. The bias voltage between the substrate and the coatings was varied from 100 mV to 3 V, depending on the conductivity of sample.
Thermo-magnetic studies were completed with a Quantum Design (QD) Physical Properties Measurement System (PPMS) using the ACMS options for measurement of the magnetization as a function of applied field and temperature. Samples were placed on a pharmaceutical gel cup mounted into a diamagnetic plastic straw.
3. Results and discussion
Initially, the samples R1 to R6 were analyzed using chemical characterization such as XRD, FTIR, XPS and elemental analysis. The changes in the chemical structure of polypyrrole and the presence of Cu2Cl(OH)3 and its degree of crystallinity were studied with respect to the reaction time. Furthermore, SEM analysis was used to correlate the structural changes in polypyrrole and Cu2Cl(OH)3 with the morphology of the resultant nanocomposite. The reaction kinetics investigated using UV-Vis spectroscopy facilitated to determine the formation rates of polypyrrole and Cu2Cl(OH)3. Finally, as illustrated by the C-AFM and magnetization results, the structural and morphological properties of individual components were reflected in the optimized conductive and magnetic properties of the final nanocomposite.3.1. XRD analysis
Fig. 1 shows the XRD patterns of R1 to R6. The crystalline peaks in R2 to R6 correspond to atacamite and clinoatacamite, which are two types of crystalline forms of Cu2Cl(OH)3, as found from power diffraction file (PDF) data. XRD pattern of R1 shows no crystalline peaks, indicating the absence of Cu2Cl(OH)3.The amorphous region in between two-theta angles of 10° and 30° in the XRD spectrum of R1 represents the polypyrrole.23 From the XRD patterns of R2 to R3, it was noticed that the intensity of the crystalline peaks increased and the area of amorphous region decreased. This indicates that the formation rate of Cu2Cl(OH)3 was higher between 2 and 3 h of reaction time. From R3 to R6, the intensity of crystalline peak of Cu2Cl(OH)3 was reduced with the formation of new amorphous region in between two-theta angles of 40° and 50°. The reduction of the crystalline region of Cu2Cl(OH)3 that occurred significantly from R4 to R6 was a clear indication that longer reaction times would affect the crystalline nature of Cu2Cl(OH)3. The dominating amorphous nature of nanocomposite in the samples R5 and R6 can be attributed to the formation of amorphous Cu2Cl(OH)3 causing the reduction in intensity of crystalline peaks. Thus, the reaction process appears to undergo probably in three major steps: (i) oxidation of pyrrole by CuCl2 leads to the formation of polypyrrole, which continuously takes place during the entire reaction, (ii) formation of crystalline Cu2Cl(OH)3, and (iii) formation of amorphous Cu2Cl(OH)3. The reaction mechanism and formation rates of polypyrrole and Cu2Cl(OH)3 were discussed in detail in the later sections of this paper.
3.2. FTIR analysis
The broad band in R1 at 3426 cm−1, shown in Fig. 2, could be attributed to N–H stretching vibrations of polypyrrole.24 This peak was slightly split into two peaks in R2 and gradually developed into sharp peaks in R3 to R6. However, hydroxyl groups of atacamite (Cu2Cl(OH)3) that happen to appear anywhere in between 3450 and 3330 cm−1 in the FTIR spectrum of atacamite were overlapped with the N–H stretching vibrations of polypyrrole.25In the spectra of all samples, the characteristic peaks of polypyrrole below 1700 cm−1 were identified.26 The band at 1478 cm−1, which corresponds to C–N stretching vibration in the pyrrole ring, was found to exhibit substantial changes with reaction time. This band gradually disappeared from R1 to R6 and a new band formation at 1404 cm−1 occurred in R5 and R6. The reduction in the intensity of C–N stretch at 1478 cm−1 relative to the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch at 1551 cm−1 indicates over-oxidation of polypyrrole and loss of conjugation.27 Changes in the shape and slight shifts in the band frequencies of C–H or C–N in-plane deformation at 1309 cm−1, the breathing vibration of the pyrrole ring at 1184 cm−1 and the C–H or N–H in-plane deformation bands at 1046 cm−1 also occurred with time, indicating probable changes in the backbone of polypyrrole. The mode of in-plane deformation vibration of N+H2, which is generally located at 1093 cm−1, is an indication of protonation of pyrrole rings and the oxidized state of polypyrrole.28 This peak was clearly visible in R1 and R2. However, the broadening of the peaks at 1184 and 1046 cm−1 in R3, R4, R5 and R6 indicates the formation of an unsymmetrical backbone of polypyrrole due to over-oxidation. As the deprotonation occurs as a result of over-oxidation, the conversion of C–N and N+H2 groups into C–OH and NH4+ takes place, which can be located around 1404 cm−1 in the spectra of R5 and R6. The strong bands below 1000 cm−1 in R1 are characteristic peaks of doped polypyrrole.29 While polypyrrole continued to remain in a doped state in R2 to R6 with subtle changes in these bands, new bands were formed in these region that are likely to correspond to Cu2Cl(OH)3.
C stretch at 1551 cm−1 indicates over-oxidation of polypyrrole and loss of conjugation.27 Changes in the shape and slight shifts in the band frequencies of C–H or C–N in-plane deformation at 1309 cm−1, the breathing vibration of the pyrrole ring at 1184 cm−1 and the C–H or N–H in-plane deformation bands at 1046 cm−1 also occurred with time, indicating probable changes in the backbone of polypyrrole. The mode of in-plane deformation vibration of N+H2, which is generally located at 1093 cm−1, is an indication of protonation of pyrrole rings and the oxidized state of polypyrrole.28 This peak was clearly visible in R1 and R2. However, the broadening of the peaks at 1184 and 1046 cm−1 in R3, R4, R5 and R6 indicates the formation of an unsymmetrical backbone of polypyrrole due to over-oxidation. As the deprotonation occurs as a result of over-oxidation, the conversion of C–N and N+H2 groups into C–OH and NH4+ takes place, which can be located around 1404 cm−1 in the spectra of R5 and R6. The strong bands below 1000 cm−1 in R1 are characteristic peaks of doped polypyrrole.29 While polypyrrole continued to remain in a doped state in R2 to R6 with subtle changes in these bands, new bands were formed in these region that are likely to correspond to Cu2Cl(OH)3.
3.3. Elemental composition analysis
As shown in Table 1, the ratio C/N was estimated to be approximately four for all the samples. The ratio of H/N in R1 and R2 was close to 3.14, which is normally observed in linear or branched polypyrrole.30 From R3 to R6, it gradually increased from 3.16 to 5.10. This increase can occur due to over-oxidation or loss of conjugation in polypyrrole as well as due to the formation of Cu2Cl(OH)3.| Label | N (mass%) | C (mass%) | H (mass%) | Empirical formula for polypyrrole | Cu (mass%) | Yield of composite (%) |
|---|---|---|---|---|---|---|
| R1 | 15.84 | 54.11 | 3.57 | C3.99H3.16N | 0.444 | 3–5% |
| R2 | 13.21 | 42.94 | 2.96 | C3.79H3.14N | 1.19 | 10–12% |
| R3 | 9.85 | 33.17 | 2.35 | C3.92H3.34N | 2.413 | 23–25% |
| R4 | 10.48 | 35.24 | 3.28 | C3.92H4.38N | 2.502 | 35–40% |
| R5 | 11.04 | 37.76 | 3.94 | C3.99H4.99N | 3.254 | 42–45% |
| R6 | 11.11 | 37.99 | 4.05 | C3.99H5.10N | 4.009 | 48–55% |
3.4. XPS analysis
The increase in N–H at 400 eV from t = 3 h (R3) to t = 4 h (R4) in Fig. 3 reflects the decrease of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N+. This can be attributed to the loss of charge on pyrrole rings or loss of conjugation due to over-oxidation.31 While the N–H percentage remained constant in the 1, 2 and 3 h samples, some of the C
N and C–N+. This can be attributed to the loss of charge on pyrrole rings or loss of conjugation due to over-oxidation.31 While the N–H percentage remained constant in the 1, 2 and 3 h samples, some of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups apparently converted to C–N+ from samples 1 to 3, probably due to the protonation and subsequent incorporation of dopant ion. From 3 h onwards, both the C
N groups apparently converted to C–N+ from samples 1 to 3, probably due to the protonation and subsequent incorporation of dopant ion. From 3 h onwards, both the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N+ groups started converting into N–H groups with apparent loss of conjugation.
N and C–N+ groups started converting into N–H groups with apparent loss of conjugation.
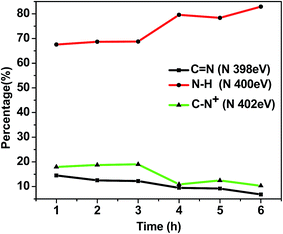 | ||
| Fig. 3 Percentages of chemical shifts of nitrogen in polypyrrole from high-resolution XPS analysis in the R1 (t = 1 h) to R6 (t = 6 h) samples. | ||
Fig. 4 shows the curve fitting of high resolution XP spectra of copper present in samples R1 to R6. The peak fitting shows four peaks of which two peaks are for core Cu 2p3/2 level consisting of two binding energy states at 932.4 and 934.6 eV, and other two peaks for its corresponding shake-up satellites at 941.4 and 944.5 eV. As shown in Fig. 5, the peak at 932.4 eV belongs to Cu(I) and the peaks, including 934.6, 941.4 and 944.5 eV, belong to Cu(II).32 Interestingly, the peak intensity at 932.4 eV, corresponding to Cu(I), was found to vary substantially from R2 to R3 and then from R5 to R6. These changes occurred most likely due to the variations in the conversion rate of CuCl to Cu2Cl(OH)3 as directed by the reaction conditions. The effect of the reaction conditions on the conversion rate is explained in detail in the next section.
 | ||
| Fig. 4 High-resolution XP spectra of copper in samples R1 to R6. The resultant components from curve fitting are also shown. | ||
 | ||
| Fig. 5 Standard curves for (a) pyrrole, (b) Cu(I), (c) the trend of pyrrole disappearance and (d) the trend of Cu(I), Cu(II) and the total of both Cu(I) and Cu(II). | ||
3.5. Reaction kinetics using UV-Vis spectrophotometry
The standard curves for pyrrole and Cu(I) are shown in Fig. 5(a) and (b), respectively, and were obtained from their corresponding stock solutions. Different concentrations of solutions of pyrrole in hexane were used to obtain the relationship between concentration and absorbance. It was found in our experiments that the maximum absorption of pyrrole between 210 and 220 nm for concentrations between 1 and ∼8 mM follows a logarithmic or exponential growth relationship between the absorbance and concentration (Fig. 5(a)). A standard procedure was followed in order to obtain standard curve for Cu(I).33 Different concentrations of Cu(II) solutions in Millipore water were made using CuCl2 and used to prepare equivalent concentrations of Cu(I) solutions by adding hydroxylamine to each of them. As shown in Fig. 6, the Cu(I) in solution forms complex with BCA and gives purple color with the corresponding absorbance value related to its concentration (Fig. 5(b)).Fig. 5(c) and (d) show the trends of concentrations of pyrrole, Cu(I), Cu(II) with time in the reaction mixture. The decay of pyrrole and Cu(II) concentrations was found to have a linear relationship with time. The concentration of Cu(I) at anytime was very small compared to the total concentration of Cu(I) and Cu(II). Therefore, the trend of Cu(II) was approximately the same as for total concentration of Cu(I) and Cu(II) in a solution. The trend of concentration of Cu(I) occurred due to the difference between the reduction rate of Cu(II) to Cu(I) and the conversion rate of Cu(I) to Cu2Cl(OH)3. Upon comparison between Fig. 5(c) and (d), it was found in this study that the disappearance of pyrrole was twice as fast as the reduction of Cu(II) to Cu(I).
Fig. 7 shows the equations of reactions involved in the formation of polypyrrole and Cu2Cl(OH)3. Redox reaction between pyrrole and CuCl2 oxidizes pyrrole to become pyrrole cation radical and reduces Cu(II) to Cu(I). This Cu(I) combines with chloride ion to form insoluble cuprous chloride (CuCl). As CuCl is not stable in the presence of oxygen and water, it gets oxidized, changes into stable form of Cu(II) and subsequently produces Cu2Cl(OH)3 depending on the availability of dissolved oxygen in water.34
3.6. SEM analysis
As shown in Fig. 8, spherical particles of the composite were observed in R1, R2 and R3 with particles size reduced from R1 to R2, and then increased from R2 to R3. The spherical particle shape gradually disappeared in between R3 and R4, and irregular and rectangular shape appeared in R4. This irregular shape was prominent in R5 and R6. Significant change in particle shape of composite occurred in between 3 and 4 h reaction time. As seen previously in the XRD results, the amorphous nature of Cu2Cl(OH)3 was predominant in R5 and R6. The chemical structural changes in polypyrrole and Cu2Cl(OH)3 clearly reflected in the morphological transformation from regular spherical and rectangular to irregular and clumped particles.3.7. Conductivity measurements
According to the literature, polypyrrole based magnetic nanocomposites can exhibit distinct conductivities depending on the type and amount of magnetic nanoparticles incorporated into the polypyrrole matrix. Polypyrrole nanocomposites containing lower amounts (∼1 w/v%) of Fe2O3, ZrO2, and SiO2 nanoparticles showed conductivities of 90, 17 and 3 S cm−1, respectively. Higher loadings of nanoparticles (∼90 wt%) caused a reduction in conductivities of the nanocomposite to approximately 10−3 S cm−1.35 Therefore, depending on the type of desired application, the required conductivity is normally obtained by adjusting the quantity of polypyrrole in the nanocomposite.In our present study, all samples (R1 to R6) showed a range of conductivity values in both C-AFM and four point probe methods, with the highest conductivity in the range of 25–33 S cm−1, displayed by R1, and lowest in the range of 0.0013–0.0006 S cm−1, displayed by R5 and R6. While I–V curves were used in C-AFM to calculate the conductivities, the voltage measured from the applied current (4.5 × 10−6 A) was used in the four-point probe method. The samples, especially R1, R2, R3, and R4, showed optimum values of conductivity in the range normally desired for good EMI and microwave shielding applications.36,37
Fig. 9 shows the AFM-current images obtained for all samples at the same scan rate of 0.5 Hz using low current sensitivity. Different DC sample voltages (bias) were required to apply in order to maintain low current sensitivity for all of them for easier comparison. The DC sample voltages applied for samples R1, R2, R3, R4, R5 and R6 were 0.1, 1, 2, 3.5, 3.8 and 3.8 V, respectively.
 | ||
| Fig. 9 AFM current images of R1, R2, R3, R4, R5 and R6 (scan rate was 0.5 Hz) with low current sensitivity. | ||
As shown in Fig. 9, the current density and magnitude over a 10 × 10 μm area gradually decreased from R1 (124.0 nA) to R6 (1.5 nA). Furthermore, the conductive areas in samples R5 and R6 were considerably smaller. The reduction in conductivity can be associated with the changes in two factors: (i) conjugation length/over-oxidation in polypyrrole and (ii) precipitation of non-conductive Cu2Cl(OH)3. As observed in FTIR previously, the ratio between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch at 1478 cm−1 and C
N stretch at 1478 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch at 1551 cm−1 appeared to be higher in the case of R1, indicating higher conjugation length in polypyrrole and therefore higher conductivity. This ratio gradually reduced from R1 to R6 reflecting the decrease in conductivity of polypyrrole. XPS results also indicated that the R5 and R6 samples contained over-oxidized polypyrrole leading to lower conjugation and as a result, lower conductivity. In addition to lowering of conductivity in polypyrrole due to chemical structural changes, the precipitation of non-conductive Cu2Cl(OH)3, as evidenced by elemental composition analysis, would also have caused the reduction in overall conductivity of nanocomposite.
C stretch at 1551 cm−1 appeared to be higher in the case of R1, indicating higher conjugation length in polypyrrole and therefore higher conductivity. This ratio gradually reduced from R1 to R6 reflecting the decrease in conductivity of polypyrrole. XPS results also indicated that the R5 and R6 samples contained over-oxidized polypyrrole leading to lower conjugation and as a result, lower conductivity. In addition to lowering of conductivity in polypyrrole due to chemical structural changes, the precipitation of non-conductive Cu2Cl(OH)3, as evidenced by elemental composition analysis, would also have caused the reduction in overall conductivity of nanocomposite.
3.8. Magnetic properties of the PPy/Cu2Cl(OH)3 nanocomposite
The magnetic behavior of any material is determined by obtaining its magnetization or magnetic moment in the presence of an applied magnetic field. Ferromagnets, known for numerous different applications such as magnetic recording devices, computer discs, transformers, and credit cards, are the materials that can retain a memory of an applied field even after it is removed. Ferrimagnets are materials that can exhibit ferromagnetic behavior only when they are in crystalline form. This ferromagnetic behavior, known as hysteresis, can be acquired by plotting a hysteresis loop, which is the relationship between magnetic moment (M) and applied magnetic field (H).38Polypyrrole based magnetic nanocomposites containing various types of nanoparticles were widely studied in the literature in terms of coercivities and magnetizations. In the work of Guo et al., nanocomposites of polypyrrole–Fe2O3 with high particle loadings of Fe2O3 nanoparticles at 20 and 50 wt% exhibited high saturation magnetizations of 29.4 and 45.1 emu g−1, respectively. However, high particle loading caused a significant reduction in conductivity of the nanocomposite.15 Polypyrrole nanocomposites prepared with manganese zinc ferrite nanoparticles were used as soft ferrimagnetic materials for radio frequency (RF) and electromagnetic interference (EMI) applications. These samples showed saturation magnetization of 0.242 emu g−1 and coercivity of 510 Oe at 10 K. Similarly, the nanocomposites of polypyrrole–Fe2O3 at small quantities of polypyrrole (0.5 and 10 wt%) showed the saturation magnetization in the range of 0.0029–0.0055 emu g−1 and coercivities of 310 and 380 Oe, indicating an increase in the magnetic properties with the inclusion of polypyrrole despite its diamagnetic property.35,39
In our current study, the magnetization measurement of the R1 and R2 samples showed that these materials possess diamagnetic properties with no hysteresis loop.38 As evidenced by the XRD and elemental analysis, the samples R1 and R2 mainly consist of polypyrrole as major component and therefore the magnetic property is predominantly influenced by the diamagnetic behavior of polypyrrole. Therefore, the samples R3, R4, R5 and R6 that exhibited hysteresis loop were only shown in Fig. 10. At low fields, a hysteretic behavior typical for magnetically ordered materials dominated in them.38 For evaluation purposes, the linear paramagnetic contribution is subtracted and the corrected M(H) for −17 kOe < H < 17 kOe range is shown in Fig. 9.
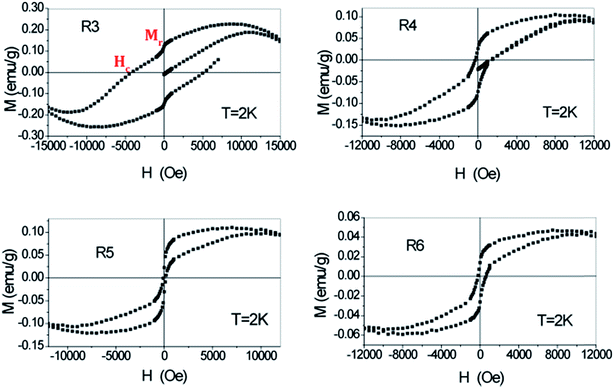 | ||
| Fig. 10 Magnetic hysteresis loops of samples R3, R4, R5 and R6 at T = 2 K after the abstraction of paramagnetic behavior. | ||
The sample R3 showed hard-ferromagnetic property with broader hysteresis loop and higher coercivity (Hc) and remanence (Mr) (shown in red in Fig. 10). As observed previously in the XRD analysis, the crystalline part of Cu2Cl(OH)3 relatively reached the highest in R3, and then gradually decreased from R3 to R6. Similarly, as it can be seen in Fig. 10, the ferromagnetic properties reduced from R3 to R6, indicating that these properties certainly stem from the crystalline part of Cu2Cl(OH)3. The approximate values of the Hc or coercive field at the temperature 2 K for samples R3, R4, R5 and R6 were 4000, 2000, 400 and 500 Oe, respectively. The Mr or the saturation magnetization for these samples R3, R4, R5 and R6 were correspondingly 53.39 emu mol−1 (0.25 emu g−1), 24.56 emu mol−1 (0.115 emu g−1), 20.92 emu mol−1 (0.1 emu g−1) and 10.25 emu mol−1 (0.05 emu g−1). From the abovementioned magnetization test results, it is clear that the ferromagnetic properties such as coercivity and remanence attain an optimum level in the nanocomposite when the reaction produces the maximum amount of crystalline Cu2Cl(OH)3. This result suggests that longer reaction times (greater than 3 h) can cause a reduction in the magnetic properties due to the formation of amorphous Cu2Cl(OH)3, as observed for the R4, R5 and R6 samples.
4. Conclusions
A polypyrrole/Cu2Cl(OH)3 nanocomposite has been in situ synthesized in a single-step via chemical oxidation of pyrrole using CuCl2 as an oxidant. The kinetics of this synthesis were studied using UV-Vis spectroscopy. In this study, it was found that while the trends of pyrrole oxidation and Cu(II) reduction were linearly dependent with time, the trend of Cu(I) followed a curvilinear due to the difference between the reduction rate of Cu(II) to Cu(I) and the conversion rate of Cu(I) to Cu2Cl(OH)3. FTIR and XPS results showed the disappearance of the CN bond and CN+ due to over-oxidation of polypyrrole. Moreover, the appearance of hydroxyl groups was observed in FTIR, confirming the gradual formation of Cu2Cl(OH)3. The high-resolution XP spectra of copper revealed that the conversion rate of Cu(I) to Cu2Cl(OH)3 would depend on the availability of dissolved oxygen. After 3 h of reaction time, the reduction in crystallinity was observed in XRD and the irregular morphology was seen in SEM. Over-oxidized polypyrrole and amorphous Cu2Cl(OH)3 resulted in lower conductivities and poor magnetization properties as the reaction progressed for longer times. Thus, from these studies, it can be concluded that by controlling the reaction conditions, the properties, such as conductivity and magnetic properties, of the polypyrrole/Cu2Cl(OH)3 nanocomposite can be optimized.Acknowledgements
The authors would like to thank the U.S. Army Research Laboratory under grant no. W911 NF-04-2-0029, W911NF-09-2-0014, W911NF-10-2-0082, and W911NF-11-2-0027 for supporting this research.References
- P. Gomez-Romero, Adv. Mater., 2001, 13, 163–174 CrossRef CAS.
- K. Rajeshwar, N. de Tacconi and C. Chenthamarakshan, Chem. Mater., 2001, 13, 2765–2782 CrossRef CAS.
- R. Gangopadhyay and A. de, Chem. Mater., 2000, 12, 608–622 CrossRef CAS.
- W. Naber, S. Faez and W. Wiel, J. Phys. D: Appl. Phys., 2007, 40, R205 CrossRef CAS.
- V. I. Krinichnyi, H. K. Roth and M. Schrodner, Appl. Magn. Reson., 2002, 23, 1–17 CrossRef CAS.
- T. Otero, Bioinspiration Biomimetics, 2008, 3, 035004 CrossRef CAS PubMed.
- K. Singh, A. Ohlan, A. Bakhshi and S. Dhawan, Mater. Chem. Phys., 2010, 119, 201–207 CrossRef CAS.
- R. Fox, V. Wani, K. Howard, A. Bogle and L. Kempel, J. Appl. Polym. Sci., 2008, 107, 2558–2566 CrossRef CAS.
- T. Vernitskaya and O. Efimov, Russ. Chem. Rev., 1997, 66, 443 CrossRef.
- N. Jadhav, C. A. Vetter and V. J. Gelling, Electrochim. Acta, 2013, 102, 28–43 CrossRef CAS.
- L. Cabrera, S. Gutierrez, M. P. Morales, N. Menendez and P. Herrasti, J. Magn. Magn. Mater., 2009, 321, 2115–2120 CrossRef CAS.
- Y. L. Luo, L. H. Fan, F. Xu, Y. S. Chen, C. H. Zhang and Q. B. Wei, Mater. Chem. Phys., 2010, 120, 590–597 CrossRef CAS.
- L. Fang, T. Y. Dai and Y. Lu, Synth. Met., 2009, 159, 2101–2107 CrossRef CAS.
- H. Xiao, W. Zhang, M. Wan and S. Fu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4446–4453 CrossRef CAS.
- Z. Guo, K. Shin, A. Karki, D. Young, R. Kaner and H. T. Hahn, J. Nanopart. Res., 2009, 11, 1441–1452 CrossRef CAS.
- X. Li, M. Wan, Y. Wei, J. Shen and Z. Chen, J. Phys. Chem. B, 2006, 110, 14623–14626 CrossRef CAS PubMed.
- J. Zhao, R. Luque, W. Qi, J. Lai, W. Gao, M. R. Hasan Shah Gilani and G. Xu, J. Mater. Chem. A, 2015, 3, 519–524 CAS.
- H. Zhang, X. Zhong, J. Xu and H. Chen, Langmuir, 2008, 24, 13748–13752 CrossRef CAS PubMed.
- K. Sunderland, P. Brunetti, L. Spinu, J. Y. Fang, Z. J. Wang and W. G. Lu, Mater. Lett., 2004, 58, 3136–3140 CrossRef CAS.
- K. Sunderland, P. Brunetti, L. Spinu, J. Fang, Z. Wang and W. Lu, Mater. Lett., 2004, 58, 3136–3140 CrossRef CAS.
- S. Yang, T. Li, B. Xu and Y. Du, J. Phys.: Condens. Matter, 2003, 15, 5629 CrossRef CAS.
- X. Zheng and E. Otabe, Solid State Commun., 2004, 130, 107–109 CrossRef CAS.
- P. M. Carrasco, H. J. Grande, M. Cortazar, J. M. Alberdi, J. Areizaga and J. A. Pomposo, Synth. Met., 2006, 156, 420–425 CrossRef CAS.
- T. Dai, X. Yang and Y. Lu, Nanotechnology, 2006, 17, 3028 CrossRef CAS.
- R. Frost, W. Martens, J. Kloprogge and P. Williams, J. Raman Spectrosc., 2002, 33, 801–806 CrossRef CAS.
- M. Omastová, M. Trchová, J. Kováová and J. Stejskal, Synth. Met., 2003, 138, 447–455 CrossRef.
- N. V. Blinova, J. Stejskal, M. Trchová, J. ProkeÅ¡ and M. R. Omastová, Eur. Polym. J., 2007, 43, 2331–2341 CrossRef CAS.
- S. Kasisomayajula, X. Qi, C. Vetter, K. Croes, D. Pavlacky and V. Gelling, J. Coat. Technol. Res., 2010, 7, 145–158 CrossRef CAS.
- T.-M. Wu, H.-L. Chang and Y.-W. Lin, Polym. Int., 2009, 58, 1065–1070 CrossRef CAS.
- B. Saunders, R. Fleming and K. Murray, Chem. Mater., 1995, 7, 1082–1094 CrossRef CAS.
- C. Malitesta, I. Losito, L. Sabbatini and P. Zambonin, J. Electron Spectrosc. Relat. Phenom., 1995, 76, 629–634 CrossRef CAS.
- C. C. Chusuei, M. A. Brookshier and D. W. Goodman, Langmuir, 1999, 15, 2806–2808 CrossRef CAS.
- M. Anwar, M. Iqbal, M. Qamar, M. Rehman and A. Khalid, World J. Microbiol. Biotechnol., 2000, 16, 135–138 CrossRef CAS.
- A. Caputo, L. J. Turbini and D. D. Perovic, J. Electron. Mater., 2010, 39, 92–96 CrossRef CAS.
- R. Gangopadhyay and A. de, Chem. Mater., 2000, 12, 608–622 CrossRef CAS.
- Ã. Z. Yavuz, M. K. Ram, M. Aldissi, P. Poddar and H. Srikanth, Synth. Met., 2005, 151, 211–217 CrossRef.
- Ã. Z. Yavuz, M. K. Ram, M. Aldissi, V. Erokhin, M. K. Ram and O. Yavuz, in The New Frontiers of Organic and Composite Nanotechnology, Elsevier, Amsterdam, 2008, pp. 435–475 Search PubMed.
- N. A. Spaldin, in Magnetic Materials: Fundamentals and Applications, Cambridge University Press, 2010, ch. 9, pp. 113–129 Search PubMed.
- P. Poddar, J. L. Wilson, H. Srikanth, S. A. Morrison and E. E. Carpenter, Nanotechnology, 2004, 15, S570 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |





