Silk fibroin as a water-soluble bio-resist and its thermal properties
Abstract
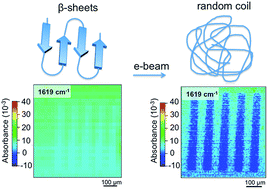
Thermal diffusivity of silk fibroin films, α = (1.6 ± 0.24) × 10−7 m2 s−1, was measured by a direct contact method. It was shown to be reduced down to ∼1 × 10−7 m2 s−1 in the crystallized phase, consistent with the multi-domain composition of β-sheet assemblies. Crystalline silk with β-sheets was made by dipping into alcohol and was used as a positive electron beam lithography (EBL) resist. It is shown by direct IR imaging of the 1619 cm−1 amide-I C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O spectral signature and 3290 cm−1 amide-A N–H stretching band that an e-beam is responsible for unzipping β-sheets, which subsequently results in exposed areas returning to a water soluble state. This makes it possible to develop a water-based biocompatible silk resist and use it in lithography applications. The general principles of protein crystallization, traceable to spectral changes in IR amide bands of silk, can be used as a guide for the creation of new protein EBL resists and to quantify the electron dose required for solubility. Foam formation and laser treatments of silk can provide new approaches in surface functionalization and fabrication of 3D bio-scaffolds.
O spectral signature and 3290 cm−1 amide-A N–H stretching band that an e-beam is responsible for unzipping β-sheets, which subsequently results in exposed areas returning to a water soluble state. This makes it possible to develop a water-based biocompatible silk resist and use it in lithography applications. The general principles of protein crystallization, traceable to spectral changes in IR amide bands of silk, can be used as a guide for the creation of new protein EBL resists and to quantify the electron dose required for solubility. Foam formation and laser treatments of silk can provide new approaches in surface functionalization and fabrication of 3D bio-scaffolds.

 Please wait while we load your content...
Please wait while we load your content...