A BiOCl/bipolar membrane as a separator for regenerating NaOH in water-splitting cells†
Xian Liu,
Xiuli Song,
Xuan Jian,
Huimin Yang,
Xiaoming Mao and
Zhenhai Liang*
College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan, Shanxi 030024, People’s Republic of China. E-mail: liangzhenhai@tyut.edu.cn
First published on 15th January 2016
Abstract
Photoconductive BiOCl has been introduced into a bipolar membrane (BPM) interlayer to prepare a BiOCl/BPM. This paper reports the use of the BiOCl/BPM composite to regenerate NaOH. Under reverse bias and sunlight irradiation conditions, the BiOCl/BPM resistance and cell voltage can be significantly decreased due to the photoconductivity of the BiOCl photocatalyst, which lead to the energy consumption decline in regenerating NaOH. Moreover, the electric field in the interlayer of the BiOCl/BPM contributes in separating the photo-generated electron–hole pairs of the BiOCl photocatalyst, thereby increasing the current efficiency of regenerating NaOH.
Introduction
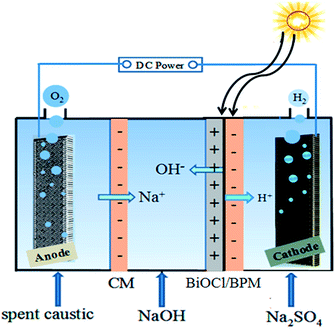
The conversion of solar energy into chemical products is considered one of the most promising approaches to sustainably satisfy escalating global energy demands.1 Bipolar membranes (BPMs) are efficient separators in water-splitting cells:2 (1) BPMs as separators in water-splitting cells could prevent the crossover of hydrogen and oxygen. (2) The BPMs could in principle be exploited to maintain a constant pH on both sides of the cell in a water-splitting system, because OH− and H+ ions are produced in the interlayer of the BPM and are consumed via the oxygen-evolution reaction (OER) and the hydrogen-evolution reaction (HER) at the anode and cathode, respectively. These pH conditions are optimal for the stability of electrodes because cathodes and anodes are generally more stable in acidic and basic media, respectively. Similarly, they are optimal for the OER and HER in terms of thermodynamics, because the OH− and H+ ions are easier to oxidize and reduce than water molecules, respectively. However, these BPMs generally have a membrane potential. Photocatalysts have been included in the interlayer of the BPMs to reduce the membrane potential by illumination.3 Bismuth oxychloride (BiOCl), as an important green photocatalyst, has aroused considerable interest due to its excellent catalytic performance.4 However, the wide band gap leads to inefficient use of solar energy.5 In our previous work, the wide band gap of BiOCl decreased to 2.30 eV when doped with iodide ions.6 Therefore, the visible-light response significantly improved. During the course of the long-time experiments, we found that the iodide ions are unstable; this will result in the efficiency always decreasing. With further research, our group prepared BiOCl visible-light catalysts with high stability by using KI and H2O2 treatment.7 In this paper, the prepared BiOCl visible-light catalysts are added into the interlayer of the BPM to form a BiOCl/bipolar membrane (BiOCl/BPM) sandwich structure. This view is based on the following considerations: (1) BiOCl, as an interlayer of a BPM, will add to the photoconductivity of BiOCl/BPM, thereby the membrane potential could be reduced. (2) The electric field in the interlayer of BPMs (reaching 105 V m−1) contributes by separating the photo-generated electron–hole pairs of BiOCl, thereby the current efficiency could be increased.This distinctive BiOCl/BPM sandwich structure is used in water-splitting cells for regenerating NaOH from spent caustic. The spent caustic generally has high pH and high elevated sodium concentrations, which is a serious threat to the environment.8 Currently, the main treatment methods of spent caustic include: acid neutralization, chemical oxidation, precipitation and biological treatment, etc.9 However, these methods generally have low treatment efficiency and expensive treatment charges, and in some cases cause secondary pollution.10 Hence, we propose a new method for regenerating NaOH from spent caustic using the BiOCl/BPM configuration, the principle is shown in Fig. 1. The device consists of three compartments, the anode chamber, central chamber, and cathode chamber, with a cation-exchange membrane layer (CM) and the BiOCl/BPM as a diaphragm. Under reverse bias and sunlight irradiation conditions, water molecules at the interlayer of the BiOCl/BPM are polarized and dissociate to form H+ and OH−. These OH− ions migrate to the central chamber, meanwhile the Na+ in the spent caustic also migrates to the central chamber, where they will combine and generate NaOH. The excitation of the carriers in modified BiOCl adds photoconductivity to the membrane and makes it a mixed electronic/ionic conductor. Therefore, the membrane resistance of the BiOCl/BPM decreases and the energy consumption for regenerating NaOH declines.
Results and discussion
The BiOCl visible light catalyst was prepared using KI and H2O2 treatment. For comparison, a BiOCl-1 sample was also prepared according to a similar method, but without the KI and H2O2 treatment (the method and process of preparation are shown in Fig. S1†). The XRD results indicate that the BiOCl and BiOCl-1 samples are well crystallized; all the diffraction peaks of the two samples were indexed to the tetragonal matlockite structure of BiOCl (JCPDS no. 73-2059) (Fig. S2†). UV-Vis DRS revealed that the BiOCl samples exhibited an obvious red shift compared to BiOCl-1. This indicates that the addition of KI/H2O2 can effectively extend the light adsorption edge into the visible light region (Fig. S3†). The XPS spectra showed that the BiOCl sample contained Cl, O and Bi (Fig. S4†).The BiOCl/BPM sandwich structure is prepared by a paste method (see ESI†). The SEM micrograph illustrates the cross section of the BiOCl/BPM (Fig. 2). As expected, the BiOCl/BPM appears to have a dense morphology without pores, and the thickness of the CM and anion-exchange membrane (AM) is 59 μm and 54 μm, respectively. The inset in the bottom left corner of Fig. 2 shows that the BiOCl/BPM has good optical transparency; this will contribute to the absorption of sunlight. The inset in the upper right corner of Fig. 2 shows that the BiOCl/BPM is water-insoluble when immersed in distilled water for 48 hours.
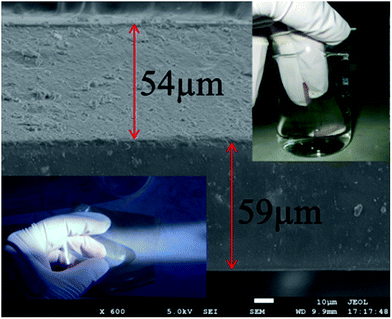 | ||
| Fig. 2 The optical images showing transparency and water-insolubility, and the SEM micrograph of the BiOCl/BPM. | ||
The current density-cell voltage measurements are obtained by placing the BiOCl/BPM in a two-compartment cell with and without sunlight irradiation, as shown in Fig. 3(a). Under sunlight irradiation, the BiOCl/BPM equipped cell exhibits lower voltage than the BiOCl-1/BPM equipped cell, suggesting that the BiOCl catalyst has higher photoconductivity than BiOCl-1. This is because the BiOCl catalyst could adsorb significantly more visible light after the KI and H2O2 treatment, and produce more electron–hole pairs from the BiOCl catalyst, thereby the photoconductivity was improved and the cell voltage was reduced significantly. The BiOCl/BPM dramatically reduced the cell voltage under no irradiation (curves b and e). This is because there is a large number of hydroxyl groups on the surface of BiOCl; hydrogen bonds between the hydrophilic groups and the water molecules are formed, which relax the bonds of the molecule in water. Moreover, these hydrophilic groups lead to improvement in the hydrophilicity of the BiOCl/BPM, more water molecules cross into the BiOCl/BPM to make up for water consumption in the interlayer, further promoting water dissociation to OH− and H+ ions. The AC impedance spectra of the BPMs are shown in Fig. 3(b). The size of the high-frequency semicircle indicates the approximate resistance. The membrane resistances of the BPMs are in the following order: BPM (no irradiation) > BiOCl-1/BPM (no irradiation) > BiOCl/BPM (no irradiation) > BiOCl-1/BPM (with irradiation) > BiOCl/BPM (with irradiation). The experimental result was consistent with Fig. 3(a). This can also be explained by the photoconductivity of BiOCl and hydrophilicity of the BiOCl/BPM.
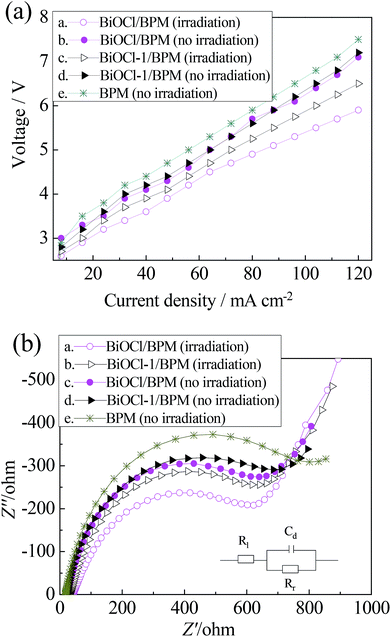 | ||
| Fig. 3 (a) Current density-cell voltage curves and (b) AC impedances with and without sunlight irradiation. | ||
In the process of NaOH recovery from spent caustic, the effects of Na2SO4 concentration in the cathode chamber on NaOH regeneration are investigated, as shown in Fig. S5 and Table S2.† The results show that NaOH yield is negligibly affected by Na2SO4 concentration, but the current efficiency firstly increases and then decreases with the decrease of Na2SO4 concentration, and reaches the maximum when the Na2SO4 concentration is 0.3 mol L−1. The effect of initial base concentration in the central chamber on NaOH regeneration is shown in Fig. S6 and Table S3.† The results show that NaOH yield and current efficiency decrease with the increase of initial base concentration in the range of 0.1–0.5 mol L−1. Thus, 0.3 mol L−1 Na2SO4 and 0.1 mol L−1 initial base will be used in the following experiments.
The current efficiency and energy consumption for regenerating NaOH using different BPMs have been investigated (for the detailed operation process and calculation methods see ESI†). Indeed, BiOCl visible light photocatalysts play an important role in NaOH recovery from spent caustic. Just as observed in our experiment which is displayed clearly in Fig. 4(a), the BiOCl/BPM will greatly improve the current efficiency, especially under sunlight irradiation. This finding could be attributed to the BiOCl visible light catalyst having higher photoconductivity as an interlayer of the BPMs and the effect of the electric field for separating electron–hole pairs. When the experiment time lasts for 1 h, the current efficiency using the BiOCl/BPM sandwich structure reaches 98% and is 8% more efficient than the BPM under sunlight irradiation. We have compared the current efficiencies with regard to NaOH recovery to other processes reported (Table 1). Kwang-Wook Kim compared the electrodialysis performance for NaOH recovery by conventional electrodialysis (ED) and electrodialysis with bipolar membranes (EDBM). The results indicated that the current efficiency of the EDBM system (about 80%) was higher than that of the ED system (about 76%).11 Jiuyang Lin reported a 80.8% current efficiency for NaOH production from wastewater using bipolar membrane electrodialysis.12 D. E. Kurath developed an electrochemical ceramic-membrane salt-splitting process to recover and recycle sodium hydroxide. The preliminary testing indicates that the sodium current efficiency reached approximately 90%.13 Ahmad Moheb showed a promising method for NaOH recovery from the waste stream of a MEROX tower, and the current efficiency reached 98%.14 We typically think that current efficiencies >80% are desirable in commercial stacks, therefore our finding is significant. There are two main reasons for the resulting difference in current efficiency under no irradiation. Firstly, on the one hand, the hydrophilic groups on the surface of BiOCl lead to improvement in the hydrophilicity of the BiOCl/BPM, which increases the water supply into the bipolar membrane to make up for water consumption in the interlayer, and promotes water dissociation into H+ and OH−. On the other hand, the excitation of the carriers in modified BiOCl adds photoconductivity to the membrane and makes it a mixed electronic/ionic conductor. The current efficiencies declined after 2 h, this is because a higher base concentration of the central chamber results in a larger osmotic pressure, which decreases the water supply into the bipolar membrane and then hampers water dissociation into H+ and OH−. Moreover, due to the so-called tunnel transport mechanism,10 the CM has a higher permeability for OH− and lower permeability for salt anions, leading to the migration of OH− from the central chamber to the anode chamber. Therefore, the NaOH yield decreases and the current efficiency declines. The experiments were repeated fifteen times to test the reusability of the system under the same conditions, the results showed that the current efficiencies and energy consumptions for regenerating NaOH using the system remained nearly unchanged, indicating that this system has good reusability.
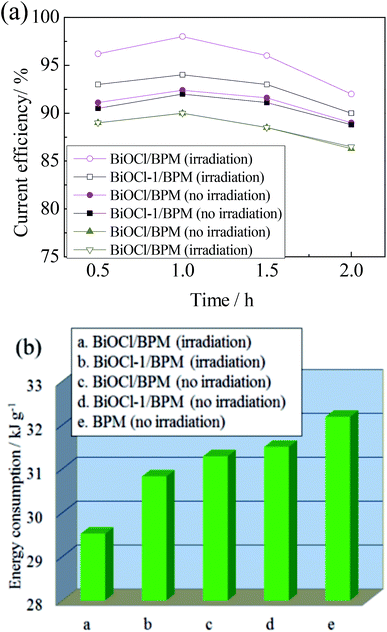 | ||
| Fig. 4 (a) The current efficiency with time for NaOH recovery and (b) the average energy consumption in the experiment after one hour. | ||
From Fig. 4(b), we can observe that the BiOCl/BPM sandwich structure exhibits the lowest energy consumption among all the BPMs for NaOH recovery. When the experiment time lasts for 1 h, the energy saved by using the BiOCl/BPM sandwich structure is about 9% compared with that of the BPM under solar irradiation. Therefore, the phenomena prove again that the BiOCl/BPM sandwich structure plays an important role in NaOH recovery from spent caustic.
Conclusions
In summary, we propose a new method for regenerating NaOH from spent caustic using a BiOCl/BPM configuration. BiOCl has excellent photoconductivity as an interlayer in BPMs, decreases membrane resistance under sunlight irradiation, and significantly reduces energy consumption. When the experiment time lasts for 1 h, the current efficiency using the BiOCl/BPM sandwich structure reached 98% and the energy saved was 9% compared with that of the BPM under sunlight irradiation. Finally, it is important to note that we have studied only NaOH regeneration from spent caustic using the BiOCl/BPM configuration. In a broader context, recovering acid from waste acid using the BiOCl/BPM configuration should be studied. As such, a series of composite membranes, such as TiO2/BPM, CdS/BPM and Cu2O/BPM may beneficial to enhance the photoconductivity of BPMs further.Acknowledgements
This work was jointly funded by the National Natural Science Foundation of China and Shenhua Group Corp (Grant no. U1261103), the National Natural Science Foundation of China (Grant no. 20771080), and the graduate education innovation projects of Shanxi provincial Department (Grant no. 2015BY19). The authors also acknowledge the Institute of Coal Chemistry, Chinese Academy of Sciences for technical assistance.Notes and references
- J. Y. Liao, D. Higgins, G. Lui, V. Chabot, X. Xiao and Z. Chen, Nano Lett., 2013, 13, 5467 CrossRef CAS PubMed; H. J. Snaith, J. Phys. Chem. Lett., 2013, 4, 3623 CrossRef; D. Wang, R. Li, J. Zhu, J. Shi, J. Han, X. Zong and C. Li, J. Phys. Chem. C, 2012, 116, 5082 Search PubMed; T. W. Kim and K. S. Choi, Science, 2014, 343, 990 CrossRef PubMed.
- N. M. Vargas-Barbosa, G. M. Geise, M. A. Hickner and T. E. Mallouk, ChemSusChem, 2014, 7, 3017 CrossRef CAS PubMed; M. B. McDonald, S. Ardo, N. S. Lewis and M. S. Freund, ChemSusChem, 2014, 7, 3021 CrossRef PubMed.
- T. Zhou, Y. Hu, R. Chen, X. Zheng, X. Chen, Z. Chen and J. Zhong, Appl. Surf. Sci., 2012, 258, 4023 CrossRef CAS; R. Chen, Y. Y. Hu, Z. Chen, X. Chen and X. Zheng, J. Appl. Polym. Sci., 2011, 122, 1245 CrossRef.
- K. Zhao, L. Zhang, J. Wang, Q. Li, W. He and J. J. Yin, J. Am. Chem. Soc., 2013, 135, 15750 CrossRef CAS PubMed; J. J. K. Zhao, X. Xiao and L. Zhang, J. Am. Chem. Soc., 2012, 134, 4473 CrossRef PubMed; X. Zhang, X. Liu, C. Fan, Y. Wang, Y. Wang and Z. Liang, Appl. Catal., B, 2013, 132, 332 CrossRef.
- J. Jiang, L. Zhang, H. Li, W. He and J. J. Yin, Nanoscale, 2013, 5, 10573 RSC; Q. Mu, Q. Zhang, H. Wang and Y. Li, J. Mater. Chem., 2012, 22, 16851 RSC; X. Wang, Q. Wang, F. Li, W. Yang, Y. Zhao, Y. Hao and S. Liu, Chem. Eng. J., 2013, 234, 361 CrossRef CAS; M. Guerrero, S. Pané, B. J. Nelson, M. D. Baró, M. Roldán, J. Sort and E. Pellicer, Nanoscale, 2013, 5, 12542 RSC.
- X. Liu, H. Yang, H. Dai, X. Mao and Z. Liang, Green Chem., 2015, 17, 199 RSC.
- X. Mao, X. Li, Y. Wang, C. Fan and H. Zhang, Chem. Eng. J., 2014, 247, 241 CrossRef CAS.
- A. Hawari, H. Ramadan, I. Abu-Reesh and M. Ouederni, J. Environ. Manage., 2015, 151, 105 CrossRef CAS PubMed; M. Graaff, M. F. M. Bijmans, B. Abbas, G. J. W. Euverink, G. Muyzer and A. J. H. Janssen, Bioresour. Technol., 2011, 102, 7257 CrossRef PubMed.
- I. B. Hariz, A. Halleb, N. Adhoum and L. Monser, Sep. Purif. Technol., 2013, 107, 150 CrossRef.
- Y. Wei, C. Li, Y. Wang, X. Zhang, Q. Li and T. Xu, Sep. Purif. Technol., 2012, 86, 49 CrossRef CAS.
- K. W. Kim, J. T. Hyun, K. Y. Lee, E. H. Lee, D. Y. Chung and J. K. Moon, Korean J. Chem. Eng., 2013, 30, 1760 CrossRef CAS.
- W. Ye, J. Huang, J. Lin, X. Zhang, J. Shen, P. Luis and B. V. Bruggen, Sep. Purif. Technol., 2015, 144, 206 CrossRef CAS.
- D. E. Kurath, K. P. Brooks, G. W. Hollenberg, D. P. Sutija, T. Landro and S. Balagopal, Sep. Purif. Technol., 1997, 11, 185 CrossRef CAS.
- N. Keramati, A. Moheb and M. R. Ehsani, Desalination, 2010, 259, 97 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and spectroscopic data. See DOI: 10.1039/c5ra20195k |
| This journal is © The Royal Society of Chemistry 2016 |