A new functionalized magnetic nanocomposite of poly(methylacrylate) for the efficient removal of anionic dyes from aqueous media†
Abstract
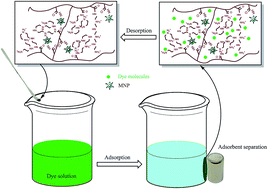
A new magnetic nano-adsorbent was synthesized via the radical polymerization of methyl acrylate on modified Fe3O4 nanoparticles, followed by its functionalization by amidation of the methyl ester groups using pentaethylenehexamine, to create active adsorption sites for removing anionic dyes from aqueous media. Physicochemical properties of the adsorbent were then characterized by SEM, TEM, XRD, FTIR, TGA and CHN analysis. The prepared nanocomposite was used as adsorbent for the removal of anionic dyes, naphthol green B and chromeazurol S, from aqueous solution and assessed in view of the kinetics and isotherm adsorption, and the effect of solution pH, contact time and initial dye concentration on adsorption capacity. Although the pH of the solution influences the surface charge of the adsorbent, in general the adsorption capacity is significant at the studied pH values. Kinetics and isotherm studies of dye adsorption indicated that the adsorption process followed pseudo-second-order kinetic and Langmuir isotherm models, respectively. The prepared adsorbent shows very high efficiency and adsorption capacity in the removal of anionic dyes, which in terms of performance are much better than most of the other adsorbents reported earlier. The maximum adsorption capacities obtained for naphthol green B and chromeazurol S were 1583 and 1348 mg g−1, respectively, at natural pH. Moreover, recycling experiments confirmed that this adsorbent could be regenerated without a significant loss of adsorption ability or weight.

 Please wait while we load your content...
Please wait while we load your content...