Design of a selective solid acid catalyst for the optimization of glucose production from Oryza sativa straw†
Amudhavalli Victora,
Indra Neel Pulidindia,
Tae Hyun Kim b and
Aharon Gedanken*ac
b and
Aharon Gedanken*ac
aDepartment of Chemistry, Bar-Ilan University, Ramat-Gan 52900, Israel. E-mail: gedanken@mail.biu.ac.il; Fax: +972-3-7384053; Tel: +972-3-5318315
bDepartment of Environmental Engineering, Kongju National University, Cheonan, Chungnam 330-717, Korea
cNational Cheng Kung University, Department of Materials Science and Engineering, Tainan 70101, Taiwan
First published on 15th December 2015
Abstract
A selective, green and fast method for the production of glucose from rice (Oryza sativa) straw is demonstrated. Aq. ammonia based pretreatment techniques played a crucial role in the removal of lignin and xylan from rice straw which in-turn accelerated glucan hydrolysis and improved the selectivity of glucose production. The cellulose isolated from rice straw is further hydrolyzed to glucose using a solid acid catalyst (activated carbon supported phosphotungstic acid, ∼40 wt% HPW/AC). Microwave irradiation of cellulose from rice straw for a short duration of 5 min at 100 °C yielded 11.2 wt% glucose relative to 8 wt% glucose produced from a hydrothermal hydrolysis process (3 h, 150 °C) with a substrate to catalyst wt/wt ratio of 1. Thus an effective biomass pretreatment (aq. ammonia–dil. H2SO4) method and an accelerated selective biomass hydrolysis process is developed.
1. Introduction
The production cost for obtaining bioethanol from rice (Oryza sativa) straw is the highest (23–26 $/GJ) relative to soy (11–15 $/GJ), palm (8–23 $/GJ), and poplar (14–17 $/GJ). It is however, comparable to that of fossil fuels (20–30 $/GJ). Optimization of the biomass conversion process is a major factor apart from labour costs and agricultural productivity in biofuel production.1 Rice straw is an abundantly available agricultural waste in Asian countries like India, China and Vietnam.2 The present rice straw based bioethanol production process cost (1.19 $/L) in Vietnam could be lowered to 0.45 $/L (plant size, 200 ML per year) by improving the pretreatment, enzyme hydrolysis, enzyme activity and productive utilization of residual biomass.3Typical composition of rice straw comprise of: cellulose (32–47%), hemicellulose (19–27%), lignin (5–24%) and ash (10–17%). High cellulose content of rice straw makes it a promising feedstock for bioethanol production for transportation application.4
Pretreatment of rice straw is necessary to remove the lignin, hemicellulose and mineral content and to isolate cellulose which upon hydrolysis yields readily fermentable glucose. The state of the art processes developed for the pretreatment of rice straw as well as the subsequent hydrolysis of pretreated rice straw to glucose are summarized in Table 1.
| Pretreatment method | Hydrolysis conditions | Yield of mono saccharides | Reference |
|---|---|---|---|
| Electron beam irradiation (50–500 kGy) followed by alkali pretreatment (3% NaOH, 5 h, 120 °C, 1 bar); lignin content decreased from 19.5 to 6.4% and cellulose content increased from 39.5 to 71.1% | Enzymatic (Celluclast 1.5 L and novozyme-188) hydrolysis at 50 °C for 24–72 h | 77 wt% total sugars (glucose (65 wt%), xylose and others) | 5 |
| Electron beam irradiation (50–500 kGy) followed by acid pretreatment (3% dil. H2SO4, 1 h, 120 °C); hemicellulose content decreased from 26.7 to 8.7%, lignin content decreased from 19.5 to 18.1% and cellulose content increased from 39.5 to 68.1% | Enzymatic (cellulase and glucosidase) hydrolysis at 50 °C for 24–72 h | 80 wt% total sugar yield (glucose selectivity – 92.7%) | 6 |
| Alkaline (NaOH 1% w/v) pretreatment for 48 h at 30 °C; solid recovery – 57.6% | Enzymatic (cellulase, xylanase and β-glycosidase) hydrolysis at 150 rpm, 50 °C for 72 h | Conversion of pretreated rice straw to reducing sugars – 92.6% | 7 |
| Hot compressed water, HCW, (140–240 °C; 10–30 min) pretreatment was effective for the removal of xylan but not lignin. Upon HCW treatment, xylan content decreased from 19% to <2% whereas lignin content increased from 18 to 40% and glucan content increased from 36 to 54% | Enzymatic (acremonium cellulase, novozyme 188, Optimash BG) hydrolysis at 45 °C for 72 h at a pH of 5 | Glucose yield – 85 wt% | 8 |
| Organosolv (75% (v/v) aq. ethanol, 1% w/w H2SO4, 180 °C, 30 min, 5% solid loading); 60% lignin removal | Enzymatic hydrolysis (cellulase and β-glucosidase), pH = 4.8, 45 °C, 72 h; 140 rpm | Glucose yield – 46.2% | 9 |
| HNO3 (0.65%, 158.8 °C, 5.86 min) pretreatment dissolved hemicellulose and yielded 86.5% xylose; moreover, the nitrate was found to be potential nutrient for improving fermentation efficiency | Enzymatic (Cellic C-Tec, novozymes, derived from Trichoderma reesei) hydrolysis at a pH 4.8, solid loading 10% w/v, 50 °C for 72 h at 170 rpm | Glycose yield – 83% (47.7 g L−1) | 10 |
| (NH4)2CO3 (20%, 80 °C, 12 h) pretreatment is effective in dissolving the amorphous components (lignin and hemi cellulose); 23.9% lignin removal and 92.3% glucan recovery | Enzymatic (Cellic C-Tec, novozymes, derived from Trichoderma reesei) hydrolysis at a pH 4.8, solid loading 5% (w/v), 50 °C for 72 h at 200 rpm | Glucose yield – 72.2% | 11 |
| Ionic liquid (cholinium argininate, [Ch][Arg]) pretreatment at 60 °C for 6 h; incubation under nitrogen and stirring; 72% lignin removal | Enzymatic (cellulase) hydrolysis at a pH – 4.8, 50 °C, 200 rpm | Glucose yield – 81% xylose yield – 26% | 12 |
| CO2 added ammonia explosion (14.3% ammonia conc.; 2.2 MPa CO2 loading, 165.1 °C, 69.8 min) pretreatment; ammonia induced swelling and removed lignin whereas CO2 caused increase in the pore size. 60% lignin removal; 80% carbohydrate recovery | Enzymatic (novozymes) hydrolysis; 50 °C, 24 h | Glucose yield 93.6% | 13 |
| Na2CO3 (0.5 M, 180 °C, 120 min) pretreatment; lignin, xylan and mineral content removed in one step process; lignin decreased from 15.5 to 8.4% and xylan decreased from 23.8 to 6.3%; glucan content increased from 40.3 to 80.1% | Enzymatic (cellulase, β-glucosidase) hydrolysis at a pH of 4.8, at 45 °C for 72 h at 100 rpm in an incubator | Glucose yield 43.5 g L−1 | 14 |
Sodium sulfite, 12% – formaldehyde (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio) at 160 °C for 1 h; selective removal of lignin (75%); sulfonation generates hydrophilic groups in the side chain of hydrophobic lignin polymer. HCHO addition raises the pH and facilitates sulfomethylation at C5 position of lignin aromatic ring 1 mole ratio) at 160 °C for 1 h; selective removal of lignin (75%); sulfonation generates hydrophilic groups in the side chain of hydrophobic lignin polymer. HCHO addition raises the pH and facilitates sulfomethylation at C5 position of lignin aromatic ring |
Enzymatic (cellulase, xylanase and β-glucosidase) hydrolysis at 50 °C, 180 rpm, 48 h, pH – 4.8 | Glucose yield 88.8% | 15 |
Na2CO3–Na2SO3 (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) pretreatment (12%, 140 °C, 60 min); presence of higher Na2CO3 facilitated hemicellulose degradation, delignification (53.4%) and conserved polysaccharide (91.4%) 1) pretreatment (12%, 140 °C, 60 min); presence of higher Na2CO3 facilitated hemicellulose degradation, delignification (53.4%) and conserved polysaccharide (91.4%) |
Enzymatic (cellulase, xylanase, glucosidase) hydrolysis at pH – 4.8, 50 °C, 180 rpm, 48 h | Glucose yield 82.7% | 16 |
| Popping (220 °C, 1.96 MPa) pretreatment increased conversion efficiency of cellulose to glucose. The chemical composition remained unchanged but a two fold increase in surface area is observed | Enzymatic (cellulase and xylanase) hydrolysis; 37 °C; 48 h | 0.394 g glucose per g biomass | 17 |
| Dil. H2SO4 (142 °C, 1.2%, 11.6 min) followed by aqueous ammonia pretreatment (20.93%, 42.7 °C, 48 h); glucan content increased from 39 to 80% as a result of removal of XMG (xylan, mannan and galactan) as well as lignin and ash | Enzymatic (cellulase, β-glucosidase) hydrolysis; pH – 4.8, 50 °C, 60 min | Glucose yield 87.24% | 18 |
| Sonication (20 kHz, 750 W, 20% amplitude, 80 °C, 50 min) assisted H2SO4 based simultaneous pretreatment and saccharification (SPS) | H2SO4 (10 wt%) based simultaneous pretreatment and saccharification | 31.8 g reducing sugar/100 g dry rice straw | 19 and 20 |
As could be noted from Table 1, even though diverse strategies were developed for effective delignification and hemicellulose removal, essentially enzymatic hydrolysis was most widely used for the conversion of pretreated rice straw to glucose except the work of Rehman et al., where H2SO4 is used for the hydrolysis.19,20 But use of mineral acids as catalyst pose problems like product separation, reactor corrosion, poor catalyst recyclability, requirement of waste effluent treatment.21 To overcome the afore mentioned issues, state of the art solid acid catalysts were developed for the hydrolysis of cellulose to glucose which was reviewed by Huang and Fu.21 Even though sulfonated carbon, polymer and magnetic solid acid based catalysts were developed, most of their applications were tested only on commercial cellulose but not on real lignocellulosic biomass. Jiang et al., have developed a sulfonated carbon catalyst for the hydrolysis of corn cob to glucose with an yield of 34 wt% under microwave irradiation (100 W, 140 °C) for 20 h.22 The main drawbacks of the process were the use of fumed H2SO4 at 120 °C for 10 h for preparing the sulfonated carbon catalyst and also the longer hydrolysis reaction times. Our approach is novel – employing a green solid acid catalyst (H3PW12O40·nH2O/activated carbon) as a substitute to either enzymes or mineral acids and using microwave irradiation for faster production of glucose. The use of heteropoly acid catalyst have several advantages including the fact that there are currently several industrial processes being operated using these solid acid catalysts.21 Moreover, in the suggested process the glucose production is accelerated with the use of microwave irradiation. Profitable bioethanol production from rice straw could be achieved by improving the biomass conversion process (pretreatment, hydrolysis and fermentation). The current work is an attempt towards developing efficient pretreatment and green hydrolysis strategies for the conversion of rice straw to glucose which is a precursor to bioethanol.
2. Experimental
2.1. Materials
Rice straw is collected from the fields of South Korea after the harvest. H3PW12O40·nH2O (HPW), HCl, H2SO4, NH3 and activated carbon (Norit® CA1 from wood, chemically activated, powder) are purchased from Sigma Aldrich Ltd., Israel. All reagents are used as received without further purification. Double distilled water is used as a solvent.2.2. Pretreatment of rice straw
The rice straw is subjected as received to three different methods of pretreatment namely, soaking in aqueous ammonia (SAA), soaking in aqueous ammonia (SAA) and subsequent hot water (SAA–hot water) treatment and soaking in aqueous ammonia with a subsequent H2SO4 treatment (SAA–H2SO4). Typical process of SAA comprise of soaking a known amount of rice straw in 15 wt% ammonia (solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio = 1
liquid ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) for 12 h at 80 °C. The other two pretreatment methods, namely, SAA–hot water and SAA–H2SO4 comprise of two stages with the reaction conditions as shown below:
12) for 12 h at 80 °C. The other two pretreatment methods, namely, SAA–hot water and SAA–H2SO4 comprise of two stages with the reaction conditions as shown below:
SAA–hot-water pretreatment: 1st stage (SAA) – 15 wt% ammonia, 80 °C, 12 h, and solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio = 1
liquid ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12; 2nd stage – hot-water, 5.0 mL min−1 of flow rate, 190 °C, 2.3 MPa.
12; 2nd stage – hot-water, 5.0 mL min−1 of flow rate, 190 °C, 2.3 MPa.
SAA–H2SO4 pretreatment: 1st stage (SAA) – 15 wt% ammonia, 80 °C, 12 h, and solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio = 1
liquid ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12; 2nd stage – dilute sulfuric acid treatment: sulfuric acid (0.2 wt%), 5.0 mL min−1 of flow rate, 170 °C, 2.3 MPa.
12; 2nd stage – dilute sulfuric acid treatment: sulfuric acid (0.2 wt%), 5.0 mL min−1 of flow rate, 170 °C, 2.3 MPa.
Solid analysis of sugar and lignin is performed following the NREL Chemical Analysis and Testing Standard procedures.24 Detailed description of the estimation of the acid soluble and acid insoluble lignin could be found elsewhere.25
2.3. Preparation of activated carbon (AC) supported tungstophosphoric acid (H3PW12O40·nH2O, HPW)
Conventional wet impregnation method is used for supporting heteropoly acid (HPW) on activated carbon.26 Typical catalyst preparation method comprise of taking 4 g of HPW in 70 mL water in a 250 mL beaker and subjecting the same for stirring using a magnetic stirrer at room temperature. To the aqueous solution of the heteropoly acid, 6 g of activated carbon is added and the content is stirred for 72 h after which the supernatant is separated from the solid component through filtration. The residual solid (∼40 wt% HPW/activated carbon) is dried in an air oven at 100 °C overnight.23 The catalyst thus obtained is characterized using SEM-EDAX (FEI Megallon 400 L microscope equipped with energy dispersive X-ray spectroscope) to evaluate the distribution of the active component (heteropoly anions) on the activated carbon surface and also by using FT-IR (Bruker Tensor 27) spectroscopy to confirm the retention of the Keggin type poly anion structure typical of heteropoly acid. The loading of the amount of HPW on the activated carbon support is evaluated using UV-Vis spectroscopy. Aq. HPW exhibits a characteristic absorption band at 256 nm due to charge transfer from bridge oxygen to tungsten in W–O–W, which was used as a measure for the quantification of HPW loaded on activated carbon (Fig. S1†).27 The amount of the HPW in the filtrate obtained in the impregnation process is subtracted from the initial amount of HPW taken, which resulted in the actual amount of HPW loaded on the support to be 39 ± 1 wt%.2.4. Hydrolysis of pretreated rice straw
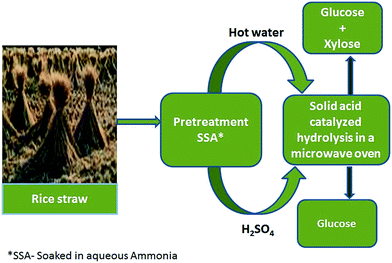
The pretreated (SSA–hot water & SSA–H2SO4) rice straw is subjected to hydrolysis in a modified domestic microwave oven (MDMWO, 2.45 GHz, 1100 W at 100% power),28 commercial microwave oven (MARS, CEM),29 and a hydrothermal reactor (cylindrical stainless steel reactor lined inside with poly tetra fluoro ethylene to resist corrosion by the acid catalyst).30 The biomass hydrolysis reaction is carried out using either conventional mineral acid (HCl, 3 M) or the solid acid catalyst (activated carbon supported tungstophosphoric acid, 40 wt% HPW/AC), under identical reaction conditions. The systematic procedure adopted for the conversion of rice straw to glucose is depicted in Scheme 1.Preliminary studies on the acid (HCl) hydrolysis of pretreated rice straw are carried out in a modified domestic microwave oven (MDMWO) with stirring facility (Fig. S2†). Typical hydrolysis batch in a MDMWO comprise of taking 0.5 g (pretreated biomass) and 20 mL 3 M HCl in a 100 mL round bottom flask and irradiating the contents for 5–10 min at 100% power.
The unreacted biomass after the acid (HCl) hydrolysis is separated from the hydrolyzate through filtration, washed with water and dried in an air oven over night at 373 K and weighed. The wt% conversion of the biomass to products is deduced from the difference in the initial (before exposure to microwave irradiation) and final (after exposure to microwave irradiation) weights of the biomass.31,32
Typical hydrolysis batch in a commercial microwave oven comprise of taking 0.125 g pretreated rice straw, 3 M HCl, 5 mL in the reaction vessel (XP-1500 plus control vessel) and irradiating the contents at 100 °C, 300 W for 1–5 min. The rice straw (pretreated) hydrolysis reaction is also evaluated using the designed solid acid catalyst (0.125 g) in the place of the mineral acid with other conditions being the same. For comparison, the acid hydrolysis of pretreated rice straw is carried out in a conventional hydrothermal reactor by taking 0.5 g biomass, 20 mL HCl (3 M) and placing the reactor in a pre-heated air oven (150 °C) for 3 h. The biomass hydrolysis reaction in the presence of solid acid catalyst is carried out by taking 0.5 g pretreated rice straw, 0.5 g 40 wt% HPW/AC, 20 mL H2O with other reaction conditions being the same as mentioned before. After the hydrolysis reaction, the residual solid mass is separated from the hydrolyzate through filtration using Whatman® (150 mm ϕ). The hydrolyzate thus obtained in each instance is qualitatively (13C NMR) and quantitatively (HPLC) analyzed for the fermentable sugars. 13C NMR spectra were recorded on a Bruker Avance DPX 300 using D2O as solvent at room temperature. HPLC analysis is carried out on a Shimadzu system with a refractive index detector (RID-10A). Chromatographic separation was carried out using a strong cation-exchange column (Aminex HPX-87H, 300 × 7.8 mm).
3. Results and discussion
3.1. Delignification of rice straw
Presence of lignin and hemicellulose in the rice straw hinders the accessibility of cellulose (exposure of cellulose surface) component by the acidic catalytic sites. Pretreatment of biomass not only solubilizes the amorphous content (lignin and hemicellulose) but also reduces the crystallinity of cellulose via pore enlargement and increase in the internal surface area.5 Among several pretreatment (biological, chemical, physical, mechanochemical) methods, alkali (ammonia, lime and NaOH) based pretreatment was found to be effective for agricultural residues.33 Alkali pretreatment is superior to other approaches, as it causes swelling of the biomass, increases the internal surface area and essentially removes the enzyme inhibitor, lignin.34 Soaking the rice straw in aq. ammonia was reported to be better than (NH4)2CO3 pretreatment for the enzymatic digestibility of the rice straw (76.5 vs. 72.2%).11In the present study, rice straw was subjected to three different pretreatments which are essentially based on aq. NH3, namely, (1) soaking in aq. NH3, (2) soaking in aq. NH3 followed by hot water treatment and (3) soaking in aq. NH3 followed by treatment with dil. H2SO4. Among the three methods, the later was found to be more effective for the removal of lignin and xylan from the rice straw yielding higher cellulose content.
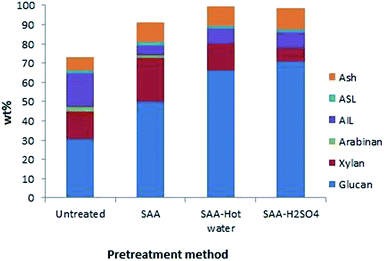
Typical composition of the rice straw before and after delignification was shown in Table S1.† Detailed mass balance of the untreated rice straw and the rice straw subjected to the three pretreatments is shown in Fig. 1.
 | ||
| Fig. 1 Mass balance of the untreated and pretreated rice straw (ASL-acid soluble lignin; AIL-acid insoluble lignin). | ||
A steady increase in the glucan (cellulose) content from 30.6 wt% to 70.6 wt% is achieved through modification of the pretreatment methods from only aq. ammonia pretreatment to aq. ammonia pretreatment followed by dil. H2SO4 treatment. Thus the pretreatment process facilitates generating cellulose rich biomass.
While soaking in aq. NH3 removed lignin from 18.7 to 9.7 wt%, subsequent treatment with dil. H2SO4 essentially enhanced xylan removal from 14.4 to 6.9 wt%. While lignin removal from rice straw accelerates the cellulose hydrolysis, xylan removal improves the selectivity of glucose production. The lignin recovered in the pretreatment process of biomass is usually regarded as a biorefinery waste. On the contrary, such lignin could be converted to value added phenolic compounds via pyrolysis. This further improves the cost effectiveness of the rice straw based bioethanol production process.36
3.2. Hydrolysis of pretreated rice straw
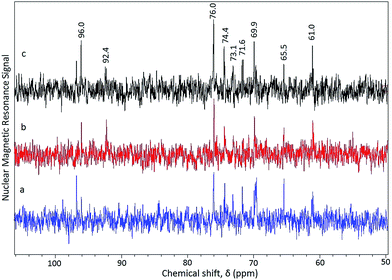 | ||
| Fig. 2 13C NMR spectra of hydrolyzate from pretreated (SAA–hot water) rice straw (a) step 1, 5 min; (b) step 2, 5 min; (c) single step, 10 min. | ||
Assignment of the signals to respective carbon nuclei of a monosaccharide was based on our previous studies.23,29 The unreacted biomass separated from the hydrolyzate through filtration was again subjected to hydrolysis using HCl in a MDMWO for 5 more minutes. A biomass conversion value of 30.4 wt% and the hydrolyzate contained both xylose and glucose (Fig. 2(b)). Thus microwave irradiation of the biomass for 10 min (in two consecutive steps each for 5 min) lead to a conversion value of 59.4 wt%. On the contrary, direct irradiation of the pretreated (SAA–hot water) rice straw for 10 min in one stretch lead to a biomass conversion value of only 28.1 wt% with the hydrolyzate comprising of both xylose and glucose (Fig. 2(c)). Thus microwave irradiation in two stages is advantageous for higher conversion of the pretreated biomass. The source of xylose in the hydrolyzate is the xylan (13.8 wt%) content present in the pretreated (SAA–hot water) rice straw. Hot water treatment following the SAA is not effective for the removal of xylan even though the lignin content is effectively removed.
Unlike SAA–hot water treated rice straw, the hydrolyzate of pretreated rice straw showed exclusive presence of glucose (Fig. 3). Pretreated (SSA–H2SO4) rice straw is hydrolyzed for 5 min in HCl under MDMWO yielding a biomass conversion value of 19 wt% and the 13C NMR spectra of the hydrolyzate showed exclusive presence of glucose (Fig. 3(a)). This selectivity towards glucose is in line with the potential of dil. H2SO4 to remove the xylan components during pretreatment.
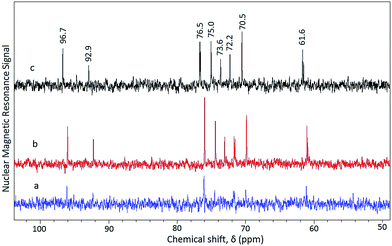 | ||
| Fig. 3 13C NMR spectra of hydrolyzate from pretreated (SAA–H2SO4) rice straw (a) step 1, 5 min; (b) step 2, 5 min; (c) single step, 10 min. | ||
The unreacted biomass is separated by filtration and reirradiated for 5 min resulting in a biomass conversion value of 35 wt% and exclusive presence of glucose is observed in the 13C NMR spectra of hydrolyzate (Fig. 3(b)). Thus a total of 54 wt% biomass conversion could be achieved in a two step microwave irradiation based hydrolysis process unlike a single step continuous irradiation of the pretreated biomass yielding only 26 wt% conversion. Contrary to the SAA–hot water pretreated rice straw, SAA–H2SO4 pretreated rice straw yielded glucose selectively. The signals at 61.6 (C6), 70.5 (C4), 73.6, 72.2 (C2), 75.0 (C3), 76.5 (C5), 92.9 (C1, α) and 96.7 (C1, β) are characteristic of glucose.32 Also the absence of signal at 65.5 (C5, β) which is a finger print for xylose presence indicate absence of xylose in the hydrolyzate. The absence of xylose in the hydrolyzate from SAA–H2SO4 pretreated rice straw signify the effectiveness of dil. H2SO4 treatment in the reduction of xylan content from 14.4 to 7 wt% (Fig. 1).
Further optimization studies of biomass hydrolysis in commercial microwave oven as well as hydrothermal reactor were carried out using SAA–H2SO4 pretreated rice straw.
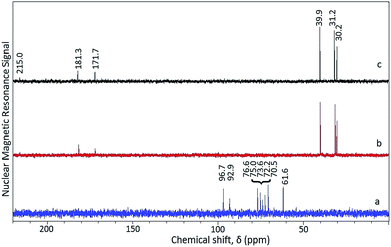 | ||
| Fig. 4 13C NMR spectra of hydrolyzate from pretreated (SAA–H2SO4) rice straw irradiated in microwave oven (MARS) for (a) 1 min; (b) 2 min; and (c) 5 min using HCl as catalyst. | ||
As the time of irradiation is increased from 1 min to either 2 or 5 min no trace of glucose is seen in the hydrolyzate as evident from the absence of signals in the region of 50–100 ppm (Fig. 4(a) and (b)). Instead, signals typical of levulinic acid were observed in the hydrolyzate obtained from 2 min (Fig. 4(b)) and 5 min (Fig. 4(c)) irradiation.
The signals at 30.2 and 39.9 ppm are characteristic of two methylene groups, one adjacent to the carbonyl and the other adjacent to the carboxyl groups. The signals at 31.2, 181.3 and 215.0 ppm correspond to methyl, carboxyl and carbonyl groups. In addition to levulinic acid, the presence of formic acid in the hydrolyzate is confirmed from the observation of the signal at 171.7 ppm.35 The presence of levulinic and formic acids in the hydrolyzate obtained in the case of pretreated rice straw irradiated for 5 min in the presence of HCl is further confirmed from 1H NMR spectrum (Fig. S3†). The presence of a singlet signal at 2.4 ppm (3H, s) and two triplets at 2.62 (2H, t) and 2.98 (2H, t) ppm confirm the presence of levulinic acid. In addition, the appearance of a singlet signal (1H, s) at 8.3 ppm is characteristic of formic acid (Fig. S3†). Thus irradiation time of 1 min at 100 °C using HCl (3 M) is optimum for the selective conversion of pretreated rice straw to glucose. But, the use of a mineral acid as catalyst for biomass conversion has several environmental as well as operational hazards. Recently, Wang et al., provided a comprehensive account of the use of solid acid catalysts for the production of glucose from cellulose. Even though, there were several additional reports on the conversion of cellulose (commercial) to glucose using solid acid catalysts, there were limited studies on the application of solid acid catalysts for the hydrolysis of real biomass feedstock.37 Keeping this in view, further hydrolysis studies of the pretreated rice straw were carried out using the solid acid catalyst (40 wt% HPW/AC) in the place of mineral acid (HCl). Interestingly, upon 5 min irradiation of the pretreated rice straw using solid acid catalyst at 100 °C, the biomass could indeed be hydrolyzed exclusively to glucose and no reaction by-products like levulinic acid or formic acid were formed. The 13C NMR spectra of the hydrolyzate obtained using solid acid catalyst showed exclusive presence of glucose (Fig. 5(a)).
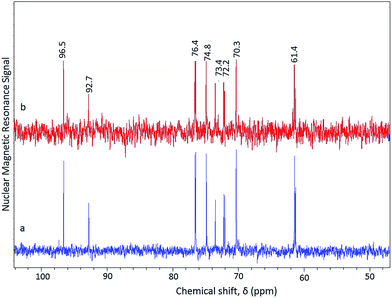 | ||
| Fig. 5 13C NMR spectra of hydrolyzate from pretreated (SAA–H2SO4) rice straw hydrolyzed using solid acid catalyst in (a) microwave oven (MARS) and (b) hydrothermal reactor. | ||
The signals at 61.4 (C6), 70.3 (C4), 72.2, 73.4 (C2), 75.0 (C3), 76.4 (C5), 92.7 (C1, α), 96.5 (C1, β) were indicative of the presence of glucose. The amount of glucose in the hydrolyzate was found to be 11.2 wt% using HPLC analysis. Irradiation of the pretreated rice straw for lower reaction time (1 and 2 min) did not result in the glucose production. Even though Onda et al., reported a much higher glucose yield (40.5 wt%) from the hydrolysis of cellulose using sulfonated carbon catalyst, reaction conditions require 48 h of ball milling of cellulose as well as longer hydrothermal reaction time (24 h) at 150 °C. Moreover, the catalyst is based on a mineral acid (H2SO4) for generating the acid sites of activated carbon surface which is not environmentally friendly.38
Apart from microwave irradiation, conventional hydrothermal method has also been evaluated for the conversion of pretreated rice straw using solid acid as catalyst. Hydrolysis of pretreated rice straw under hydrothermal conditions (150 °C, 3 h) using ∼40 wt% HPW/AC resulted in the selective production of glucose. The hydrothermal reaction product showed similar 13C NMR peak pattern (Fig. 5(b)) to that obtained in a microwave reactor (Fig. 5(a)).
Apart from glucose (characteristic signals in the region of 50–100 ppm), no other signals typical of common by-products like levulinic acid and formic acid were observed indicating the selective nature of the solid acid catalyst for the conversion of pretreated rice straw to glucose. The yield of glucose in the hydrolyzate from the hydrothermal reaction was found to be 8 wt% using HPLC analysis.
It is clear that the yield of glucose is higher and also the reaction time is shorter and the reaction temperature is lower in the microwave irradiation based biomass hydrolysis compared to the conventional hydrothermal reaction. Thus under modest reaction conditions (100 °C, 5 min), glucose could be produced from the pretreated rice straw using the solid acid catalyst offering a green pathway for biomass conversion. The hydrolyzate obtained under microwave irradiation (MARS) is further analyzed by UV-Vis analysis for possible leaching of the heteropoly acid into the medium during the reaction. No absorption band characteristic of HPW is observed in the hydrolyzate indicating the stability of the HPW/AC catalyst (Fig. S1†).
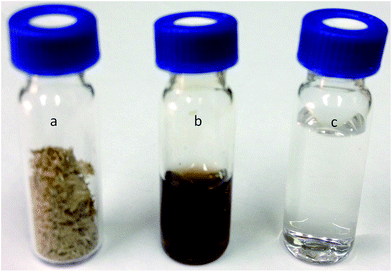
Under similar microwave reaction conditions, use of mineral acid catalyst lead to the formation of levulinic acid. The appearance of the reaction product of the hydrolysis of pretreated rice straw using solid acid catalyst vs. mineral acid could be seen in Fig. 6. Deep brown colouration (Fig. 6(b)) is typical of levulinic acid formation (with HCl as catalyst) whereas the reaction product using solid acid catalyst is transparent and colourless (Fig. 6(c)) as expected of a glucose solution.
3.3. Characterization of the solid acid catalyst, ∼40 wt% HPW/AC
FT-IR spectra of the solid acid catalyst (∼40 wt% HPW/AC) before and after the biomass hydrolysis reaction in a microwave oven (MARS) is shown in Fig. 7.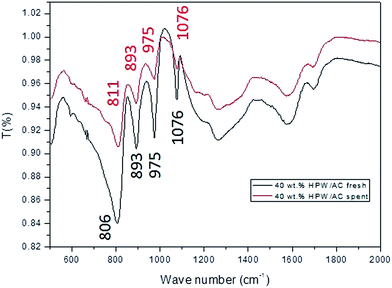 | ||
| Fig. 7 FT-IR spectra of (a) fresh and (b) spent catalyst (∼40 wt% HPW/AC) used for the hydrolysis of rice straw under micro wave irradiation. | ||
The presence of four consecutive bands in the region of 800–1100 cm−1 is a finger print of the Keggin type polyanion and they are a result of the four kinds of metal-oxygen bonds present in the heteropoly anion [PW12O40]3−.26 Interestingly, similar features were also observed in the case of the spent catalyst indicating that the structure of the active component at the molecular level remained unperturbed even after microwave irradiation.
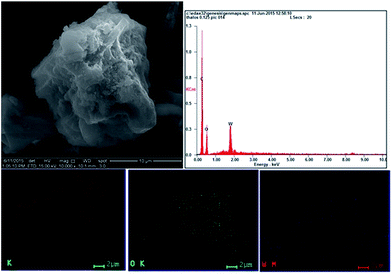
The solid acid catalyst, before and after the biomass hydrolysis reaction under microwave irradiation (MARS), was further characterized using SEM-EDAX with elemental mapping. The morphology of the ∼40 wt% HPW/AC could be seen in the SEM (Fig. 8).
Activated carbon particle of irregular shape could be viewed upon which the active component H3PW12O40 is homogeneously distributed. The homogeneous distribution of the heteropoly acid could be observed from the elemental mapping in Fig. 8. As expected W (in blue colour) and oxygen (green) were distributed upon carbon (red colour) surface. Interestingly, even in the spent catalyst similar elemental distribution is observed (Fig. S4†) indicating that the poly anion, [PW12O40]3− is strongly adhered to the activated carbon surface which remained bound to the surface even after the biomass hydrolysis reaction.
This result (SEM-EDAX) is in concurrence with the FT-IR (Fig. 7) analysis of the solid acid catalyst. Thus the solid acid catalyst designed is potential (active and selective) for the conversion of pretreated rice straw to glucose. Owing to the industrial demand for green solid acid catalysts for biomass conversion stringent efforts are being carried out not only for glucose production but also for a variety of catalytic reactions. Pesaresi et al., developed Cs-doped silico tungstic acid catalyst for the production of biodiesel via transesterification of C4 and C8 glycerides.39 Fraile et al., developed a sulfonated carbon catalyst for the esterification of palmitic acid with methanol.40 Ngee et al., designed a novel sulfated mesoporous niobium oxide catalyst for the conversion of a variety of sugars (fructose, sucrose, cellobiose and inulin) into a valuable fuel precursor, 5-hydroxy methyl furfural (5-HMF).41 Pent et al., reported a sulfonated two-dimensional covalent organic frame work synthesized from the condensation of 1,3,5-triformyl phloroglucinol and 2,5-diaminobenzenesulfonic acid (THF-DABA), which efficiently catalyzed the conversion of fructose to 5-HMF (97% yield).42
Effective pretreatment of rice straw, ease of synthesis of the catalyst (HPW/AC), environmental friendliness, stability of the catalyst under microwave irradiation conditions, fast and selective production of glucose from pretreated rice straw were the salient features of the current process.
4. Conclusions
Abundantly available biomass, rice straw, is pretreated by soaking in aqueous ammonia followed by treating with dil. H2SO4. Reaction with aq. NH3 facilitated the removal of lignin whereas treatment with dil. H2SO4 effectively removed hemicellulose (xylan). The pretreated rice straw was further hydrolyzed using two different class of catalysts: conventional mineral acid and solid acid catalyst. Use of solid acid catalyst (∼40 wt% tungsto phosphoric acid/activated carbon) under microwave irradiation (5 min, 100 °C) yielded 11.2 wt% glucose exclusively. No by-products like levulinic and formic acids which were obtained in the case of mineral acid were observed when solid acid catalyst is used. For comparison, conventional hydrothermal method (150 °C, 3 h) of heating was also used for the hydrolysis of pretreated rice straw which resulted in the selective production of glucose (8 wt%). Microwave based hydrolysis using solid acid catalyst is particularly advantageous owing to shorter reaction time, lower reaction temperature, higher and selective glucose yield. The structural integrity of the solid acid catalyst is retained even after the microwave irradiation.Acknowledgements
Gedanken thanks the Ministry of Science and Technology (MoST) for the research grants 3-9802, 3-99763 and the Israel Science Foundation (ISF) for supporting the research via a grant 598/12. Grateful thanks are due to Dr Alexander Varvak, Bar Ilan university, for the valuable assistance in the HPLC analysis.References
- J. V. Eijck, B. Batixzirai and A. Faaij, Appl. Energy, 2014, 135, 115–141 CrossRef.
- L. D. Escribà and M. Porcar, Biofuels, Bioprod. Biorefin., 2010, 4, 154 CrossRef.
- N. Q. Diep, K. Sakanishi, N. Nakagoshi, S. Fujimoto and T. Minowa, J. Renewable Energy, 2015, 74, 456 CrossRef.
- P. Binod, R. Sindhu, R. R. Singhania, S. Vikram, L. Devi, S. Nagalakshmi, N. Kurein, R. K. Sukumaran and A. Pandey, Bioresour. Technol., 2010, 101, 4767 CrossRef CAS PubMed.
- D. Y. Kim, B. M. Lee, Y. J. Lee, P. H. Kang and J. P. Jeun, J. Korean Soc. Appl. Biol. Chem., 2014, 57(5), 591 CrossRef.
- B. M. Lee, J. Y. Lee, P. H. Kang, S. K. Hong and J. P. Jeun, Appl. Biochem. Biotechnol., 2014, 174, 1548 CrossRef CAS.
- E. Cabrera, M. J. Munoz, R. Martin, I. Caro, C. Curbelo and A. B. Diaz, Bioresour. Technol., 2014, 167, 1 CrossRef CAS.
- G. Yu, S. Yano, H. Inoue, S. Inoue, J. Wang and T. Endo, Appl. Biochem. Biotechnol., 2014, 174, 2278 CrossRef CAS.
- H. Amiri, K. Karimi and H. Zilouei, Bioresour. Technol., 2014, 152, 450 CrossRef CAS.
- I. Kim, B. Lee, J. Y. Park, S. A. Choi and J. I. Han, Carbohydr. Polym., 2014, 99, 563 CrossRef CAS.
- I. Kim, B. Lee, D. Song and J. I. Han, Bioresour. Technol., 2014, 166, 353 CrossRef CAS PubMed.
- X. D. Hou, J. Xu, N. Li and M. H. Zong, Biotechnol. Bioeng., 2015, 112(1), 65 CrossRef CAS.
- Y. L. Cha, J. Yang, J. W. Ahn, Y. H. Moon, Y. M. Yoon and G. D. Yu, Bioprocess Biosyst. Eng., 2014, 37, 1907 CrossRef CAS.
- S. M. Salehi, K. Karimi, T. Behzad and N. Poornejad, Energy Fuels, 2012, 26, 7354–7361 CrossRef CAS.
- F. Gu, W. Wang, L. Jing and Y. Jin, Bioresour. Technol., 2013, 142, 218 CrossRef CAS.
- L. Yang, J. Cao, J. Mao and Y. Jin, Ind. Crops Prod., 2013, 43, 711 CrossRef CAS.
- S. G. Wi, I. S. Choi, K. H. Kim, H. M. Kim and H. J. Bae, Biotechnol. Biofuels, 2013, 6, 166 CrossRef PubMed.
- S. B. Kim, S. J. Lee, J. H. Lee, Y. R. Jung, L. P. Thapa, J. S. Kim, Y. Um, C. Park and S. W. Kim, Biotechnol. Biofuels, 2013, 6, 109 CrossRef CAS.
- M. S. U. Rehman, I. Kim, K. H. Kim and J. I. Han, Int. J. Environ. Sci. Technol., 2014, 11, 543 CrossRef CAS.
- M. S. U. Rehman, M. A. Umer, N. Rashid, I. Kim and J. I. Han, Ind. Crops Prod., 2013, 49, 705 CrossRef.
- Y. B. Huang and Y. Fu, Green Chem., 2013, 15, 1095 RSC.
- Y. Jiang, X. Li, X. Wang, L. Meng, H. Wang, G. Peng, X. Wang and X. Mu, Green Chem., 2012, 14, 2162 RSC.
- V. B. Kumar, I. N. Pulidindi and A. Gedanken, J. Renewable Energy, 2015, 78, 141 CrossRef CAS.
- NREL, Chemical Analysis and Testing Laboratory Analytical Procedures (CAT), National Renewable Energy Laboratory, Golden.
- T. H. Kim, Korean J. Chem. Eng., 2012, 29(1), 82 CrossRef CAS.
- I. N. Pulidindi, Development and exploitation of carbon materials from plant sources, Ph. D. Thesis, Indian Institute of Technology, Madras, 2009.
- M. Sliwa, K. Samson, M. Ruggiero-Mikolajczyk, A. Zelazny and R. Grabowski, Catal. Lett., 2014, 144, 1884 CrossRef CAS.
- I. N. Pulidindi, B. B. Kimchi and A. Gedanken, J. CO2 Util., 2014, 7, 19 CrossRef CAS.
- A. Victor, I. N. Pulidindi and A. Gedanken, J. Environ. Manage., 2015, 162, 215 CrossRef CAS.
- A. Victor, I. N. Pulidindi and A. Gedanken, RSC Adv., 2014, 4, 44706 RSC.
- I. N. Pulidindi, R. H. Mariana, M. Patricia and A. Gedanken, J. Fundam. Renewable Energy Appl., 2014, 4(2), 143, DOI:10.4172/2090-4541.1000143.
- I. N. Pulidindi, B. B. Kimchi and A. Gedanken, J. Renewable Energy, 2014, 71, 77 CrossRef CAS.
- D. J. Seo and A. Sakoda, Biomass Bioenergy, 2014, 71, 47 CrossRef CAS.
- A. Das, T. Paul, A. Jana, S. K. Halder, K. Ghosh, C. Maity, P. K. D. Mohapatra, B. R. Pati and K. C. Mondal, Ind. Crops Prod., 2013, 46, 217 CrossRef CAS.
- A. Szabolcs, M. Molnár, G. Dibó and L. T. Mika, Green Chem., 2013, 15, 439 RSC.
- K. A. Jug, S. H. Woo, S. R. Lim and J. M. Prak, Chem. Eng. J., 2015, 259, 107 CrossRef.
- J. Wang, J. Xi and Y. Wang, Green Chem., 2015, 17, 737 RSC.
- A. Onda, T. Ochi and K. Yanagisawa, Green Chem., 2008, 10, 1033–1037 RSC.
- L. Pesaresi, D. R. Brown, A. F. Lee, J. M. Montero, H. Williams and K. Wilson, Appl. Catal., A, 2009, 360, 50 CrossRef CAS.
- J. M. Fraile, E. Garcia-Bordeje and L. Roldan, J. Catal., 2012, 289, 73 CrossRef CAS.
- E. L. S. Ngee, Y. Gao, X. Chen, T. M. Lee, Z. Hu, D. Zhao and N. Yan, Ind. Eng. Chem. Res., 2014, 53, 14225 CrossRef CAS.
- Y. Peng, Z. Hu, Y. Gao, D. Yuan, Z. Kang, Y. Qian, N. Yan and D. Zhao, ChemSusChem, 2015, 8, 3208 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra20121g |
| This journal is © The Royal Society of Chemistry 2016 |