A scandium-doped manganate anode for a proton-conducting solid oxide steam electrolyzer†
Abstract
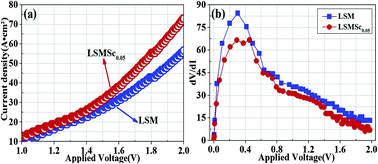
Perovskite La0.8Sr0.2MnO3−δ is widely used as an anode for proton-conducting solid oxide steam electrolyzers; however, the insufficient electro-catalytic activity still restricts the electrochemical steam oxidation activity. In this work, catalytically-active scandium is doped into the B-site of the manganate La0.8Sr0.2Mn1−xScxO3−δ (x = 0–0.1) to enhance the electrocatalytic performance. Combined characterizations of XRD, TEM, XPS, SEM and EDS confirm the successful partial replacement of Mn by Sc in the B-site. The doping of Sc remarkably improves ionic conductivity while accordingly decreases electronic conductivity. The electrocatalytic activity has been greatly improved and the composition of La0.8Sr0.2Mn1−xScxO3−δ with x = 0.05 has demonstrated the best electrode polarization performance. The faradic efficiency is significantly enhanced to as high as 80% for La0.8Sr0.2Mn1−xScxO3−δ (x = 0.05) in a proton conducting solid oxide electrolyzer in contrast to a cell with traditional LSM anode for high temperature steam electrolysis.

 Please wait while we load your content...
Please wait while we load your content...