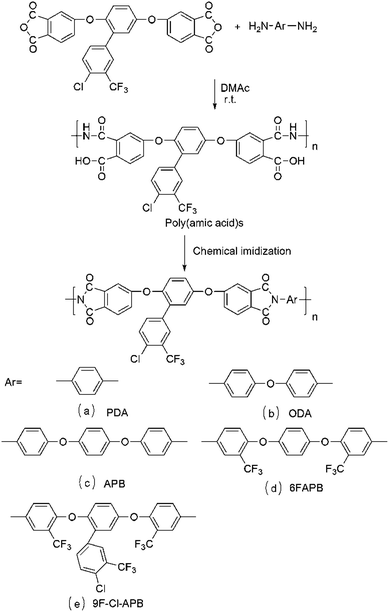
Novel soluble poly(ether imide)s with trifluoromethyl and chloride pendant groups for optical materials†
Yu Liua,
Chang Gaoa,
Linjiu Xiaoa,
Ying Xiea,
Yu Wangb and
Wenze Li*a
aCollege of Applied Chemistry, Shenyang University of Chemical Technology, Shenyang 110142, China. E-mail: liwenze@syuct.edu.cn
bDepartment of Chemistry, Queen's University, Kingston, Ontario K7L 3N6, Canada
First published on 3rd February 2016
Abstract
A new bis(ether dianhydride) monomer [4,4′-(2-(3′-trifluoromethyl-4′-chlorophenyl)-1,4-phenylenedioxy)-diphthalic anhydride (3)] was designed and prepared through a multistep reaction sequence. The new monomer 3 was polymerized with typical diamines, such as PDA, ODA, APB, 6FAPB and 9F–Cl-APB, using a two-step chemical imidization method to obtain the corresponding poly(ether imide)s (PEIs) (4a–4e). All the PEIs had high glass transition temperatures, excellent thermal stability, good solubility, and could be made into transparent and flexible films. The cast films showed UV-vis absorption edges at 346–369 nm, low birefringences of 0.0052–0.0131, low dielectric constants of 2.69–2.80, and low water uptakes of 0.30–0.52 wt%. Based on the characterization results, a series of optical materials with tunable refractive indices were obtained by copolymerization in the range of 1.6217–1.5523 at 650 nm.
Introduction
Aromatic polyimides (PI) are widely known as high performance materials originally developed for the aerospace industry. High thermal stability combined with chemical resistance and excellent electrical and mechanical properties make PIs attractive as versatile high performance materials for a variety of applications such as composites, coatings, electric materials and membranes.1,2 High optical transparency is one of the most desirable properties of PI materials for a wide diversity of applications like flexible solar cell arrays, or micro-optical devices in the field of displays and waveguides.3–7 Despite their widespread use, their rigid backbones and strong intermolecular interactions restrict their applications in some fields. Most of them have high melting or softening temperatures, and limited solubility in most organic solvents. Another shortcoming is the pale yellow or deep brownish-yellow color of PI films due to their highly conjugated aromatic structures and the formation of intermolecular charge-transfer complexes (CTC) and electronic polarization interactions.8,9To solve these problems, considerable research effort has been focused on the synthesis of soluble and transparent PIs in a fully imidized form without deterioration of their excellent properties. In the past decades, many attempts have been made to improve the optical properties of PIs by incorporating fluorine, chlorine or sulfone groups, introducing unsymmetrical and bulky pendent units, as well as adopting alicyclic moieties in the polymer structure.10–14 Therefore, the development of soluble PIs has attracted major interest in the research of aromatic polymers. Fortunately, progress has been achieved with considerable efforts on improving the capacity and the solubility of PIs through structure design, modification of aromatic dianhydride and diamine monomers.15,16 Among many approaches, the incorporation of trifluoromethyl (CF3) groups into polymer chains is considered an effective means of realizing soluble and transparent PIs without deteriorating their excellent properties, not only because the bulky CF3 groups disturb the interactions and chain packing between the polymer chains, but also because the C–F bond is one of the strongest single bonds. Thus, this method can optimize the trade-off between processability and color properties of aromatic PIs.17–22
At present, most dissoluble aromatic PIs containing fluorine were prepared by using different kinds of aromatic diamines containing fluorine rather than fluorinated aromatic dianhydrides, except for 6FDA, which is currently commercially available.23–26 However, the widespread applications and commercialization of 6FDA are also hampered by the synthetic procedure. Our previous research on fluorinated aromatic PIs demonstrated that the incorporation of bulky trifluoromethyl groups into diamines and dianhydrides resulted in an enhanced solubility and a lower dielectric constant, as well as an improved optical transparency.27–30
Besides the carbon–fluorine bonds (C–F bonds), the introduction of carbon–chlorine bonds (C–Cl bonds) can also improve the polymer optical properties. Because the C–Cl bond is reported to have low loss at 1310 and 1550 nm (optical telecommunication region) like the C–F bond based on the calculation, and has larger polarizability than the C–F bond, incorporation of the C–Cl bond into the polymer backbone may yield good refractive index controllability without additional optical loss.31,32 In addition, the introduction of bulky moieties (CF3 and Cl groups) into the polymers would increase the chain-packing distances and decreased intermolecular interactions further. It has been demonstrated that the introduction of bulky pendant groups into polyimide backbones resulted in enhanced solubility and optical transparency. Thus, chlorofluorinated polyimides for copolymerization for controlling the refractive index can be a potential candidate for optical application.
As part of our continuing efforts to gain colorless and soluble PIs with high thermal stability and a wide application potential in optoelectronics, novel bis(ether dianhydride) [4,4′-(2-(3′-trifluoromethyl-4′-chlorophenyl)-1,4-phenylenedioxy)-diphthalic anhydride (3)] was designed and prepared. A series of soluble poly(ether imide)s (PEI) (4a–4e) were prepared by polycondensation of bis(ether dianhydride) (3) with various aromatic diamines (a–e). From the characterization results, the prepared PEIs exhibited good solubility, excellent thermal and mechanical properties, low water uptake, low dielectric constants and high optical properties, including high optical transparency, low birefringence, and low NIR absorptions loss at the communication windows. Moreover, a series of PEIs optical materials with tunable refractive indices were obtained by copolymerization.
Experimental
Materials
The (3-fluoromethyl-4-chlorophenyl)-1,4-hydroquinone was synthesized according to the literature.33 4-Nitrophthalodinitrile (TCI), potassium carbonate (K2CO3, Aldrich) and acetic anhydride were obtained from commercial sources used as received. N,N-Dimethylformamide (DMF, Aldrich), N,N-dimethylacetamide (DMAc, Aldrich) and pyridine (Py, Aldrich) were purified by distillation under reduced pressure over calcium hydride and stored over 4 Å molecular sieves. 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) (TCI), p-phenylenediamine (PDA) (TCI), 4,4′-oxydianiline (ODA) (TCI) and 1,4-bis(4-aminophenoxy)benzene (APB) (TCI) were used without further purification. 1,4-Bis(4-amino-2-trifluoromethylphenoxy)benzene (6FAPB) and 1,4-(4-amino-2-trifluoromethylphenoxy)-2-(3′-trifluoromethyl-4′-chlorophenyl) benzene (9F–Cl-APB) was synthesized in our laboratory according to the previously reported.34Measurements
Gel permeation chromatograms (GPC) were obtained on a Waters 410 instrument with N,N-dimethylformamide (DMF) as an eluent at a flow rate of 1 mL min−1 using polystyrene as a standard. FTIR spectra were measured on a Nicolet Impact 410 Fourier transform infrared spectrometer. 1H NMR spectra were recorded on a Bruker 510 NMR spectrometer (500 MHz) with tetramethyl silane as a reference. Elemental analysis was performed on an Elemental Analyses MOD-1106. Inherent viscosity was determined on an Ubbelohde viscometer in thermostatic container with the polymer concentration of 0.5 g dL−1 in DMAc at 25 °C. Differential scanning calorimetry (DSC) measurements were performed on a Mettler Toledo DSC 821e instrument at a heating rate of 20 °C min−1 under nitrogen. Thermal gravimetric analyses (TGA) were determined in nitrogen atmosphere using a heating rate of 10 °C min−1 and polymers were contained within open aluminum pans on a PERKIN ELMER TGA-7. The refractive indices of the polymer were studied using a Gaertner L116B spectroscopic ellipsometer. The birefringences of the polymer films, at the 650 nm wavelength, were determined from coupling angles of TE (transverse electric) of TM (transverse magnetic) optical guided modes with a gadolinium gallium garnet (GGG) prism. UV-visible transparency was measured by a Shimadzu UV 2501-PC spectrophotometer. Wide-angle X-ray diffraction (WAXD) measurements were made at room temperature using a Rigaku/max-rA diffractometer equipped with a Cu Kα radiation source. The mechanical tests in tension were carried out using a SHIMADZU AG-I at a constant crosshead speed of 10 mm min−1. The equilibrium moisture absorption was determined by weighing the changes in vacuum-dried film specimens before and after immersion in deionized water at 25 °C for 3 days. The photographs of the PIs films were taken by Canon EOS 6D.Monomer synthesis
As shown in Scheme 1, the bis(ether dianhydride) monomer 3 was synthesized from the nitro-displacement reaction of 4-nitrophthalodinitrile with (3-fluoromethyl-4-chlorophenyl)-1,4-hydroquinone in N,N-dimethylformamide (DMF) in the presence of potassium carbonate as the base, followed by the alkaline hydrolysis of the intermediate bis(ether dinitrile)s and the cyclodehydration of the resulting bis(ether diacid)s. The synthesis details have been described in a previous article.28 The characterization of the target bis(ether anhydride) (3) is listed as follows:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1272 (C–O–C), 1120 (C–F). 1H NMR (500 MHz, DMSO-d6, δ, ppm): 13.14 (br, 4H, –COOH), 8.21 (d, J = 7.4 Hz, 2H), 8.06 (s, H), 7.81 (d, J = 9.3 Hz, 1H), 7.70 (t, J = 8.1 Hz, 2H), 7.35 (d, J = 9.5 Hz, 2H), 7.22 (s, 2H), 7.06 (s, 2H) (ESI, Fig. S2†).
O), 1272 (C–O–C), 1120 (C–F). 1H NMR (500 MHz, DMSO-d6, δ, ppm): 13.14 (br, 4H, –COOH), 8.21 (d, J = 7.4 Hz, 2H), 8.06 (s, H), 7.81 (d, J = 9.3 Hz, 1H), 7.70 (t, J = 8.1 Hz, 2H), 7.35 (d, J = 9.5 Hz, 2H), 7.22 (s, 2H), 7.06 (s, 2H) (ESI, Fig. S2†).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1772 (sym C
O), 1772 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1276 (C–O–C), 1120 (C–F). 1H NMR (500 MHz, CDCl3, δ, ppm): 7.97 (d, J = 8.2 Hz, 1H), 7.86 (d, J = 6.5 Hz, 2H), 7.64 (d, J = 8.2 Hz, 1H), 7.58 (dd, J = 8.4, 2.2 Hz, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.39 (dd, J = 8.4, 2.2 Hz, 1H), 7.37–7.34 (m, 1H), 7.33 (s, 1H), 7.31 (d, J = 9.3, 2.5 Hz, 2H), 7.29 (dd, J = 9.3, 2.5 Hz, 2H). Elem. anal. calcd for C29H12ClF3O8 (580.85): C 59.97, H 2.08. Found: C 60.02, H 2.03.
O), 1276 (C–O–C), 1120 (C–F). 1H NMR (500 MHz, CDCl3, δ, ppm): 7.97 (d, J = 8.2 Hz, 1H), 7.86 (d, J = 6.5 Hz, 2H), 7.64 (d, J = 8.2 Hz, 1H), 7.58 (dd, J = 8.4, 2.2 Hz, 1H), 7.51 (d, J = 2.1 Hz, 1H), 7.39 (dd, J = 8.4, 2.2 Hz, 1H), 7.37–7.34 (m, 1H), 7.33 (s, 1H), 7.31 (d, J = 9.3, 2.5 Hz, 2H), 7.29 (dd, J = 9.3, 2.5 Hz, 2H). Elem. anal. calcd for C29H12ClF3O8 (580.85): C 59.97, H 2.08. Found: C 60.02, H 2.03.Polymer synthesis
Utilizing monomer 3 as dianhydride, five kinds of PEIs were synthesized by polycondensation with diamine monomers PDA, ODA, APB, 6FAPB and 9F–Cl-APB, respectively. The resulting PEIs were abbreviated to 4a–4e, respectively.In a typical experiment, PEI-4c was derived from dianhydride 3 and diamine APB (c). To a 50 mL thoroughly dried three-neck flask, 2 mmol dianhydride monomer 3 was dissolved in 8 mL of DMAc. To this dianhydride solution, 2 mmol diamine APB and an additional 5 mL of DMAc were added. The mixture was stirred for 24 h at room temperature under nitrogen to yield a viscous poly(amic acid) PAA solution. Chemical imidization was performed via the addition of 1.5 g triethylamine and 4.5 g of acetic anhydride into the PAA solution at ambient temperature. The mixture was stirred at 60 °C for 6 h to yield a PEI solution. Then, the solution was poured into anhydrous ethanol to give a precipitate and washed thoroughly with anhydrous ethanol before drying at 80 °C in vacuum. IR (KBr, cm−1): 1779 and 1721 (asymmetric, symmetric imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch), 1619–1480 (aromatic C
O stretch), 1619–1480 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch), 1382 (C–N stretch), 1100–1300 (C–O and C–F stretching). 1H NMR (500 MHz, CDCl3, δ, ppm): 7.97 (d, 1H), 7.86 (d, 2H), 7.64 (d, 1H), 7.55 (s, 1H), 7.50 (dd, 2H), 7.37 (dd, 4H), 7.32 (s, 1H), 7.25 (ddd, 4H), 7.12 (m, 8H).
C stretch), 1382 (C–N stretch), 1100–1300 (C–O and C–F stretching). 1H NMR (500 MHz, CDCl3, δ, ppm): 7.97 (d, 1H), 7.86 (d, 2H), 7.64 (d, 1H), 7.55 (s, 1H), 7.50 (dd, 2H), 7.37 (dd, 4H), 7.32 (s, 1H), 7.25 (ddd, 4H), 7.12 (m, 8H).
The other PEIs (4a, 4b, 4d and 4e) (Scheme 2) were prepared by a similar procedure, adding the corresponding diamine (PDA, ODA 6FAPB and 9F–Cl-APB) instead of APB.
Results and discussion
Synthesis and characterizations of the monomer
The bis(ether anhydride) monomer 3 was prepared by a three-step reaction sequence, as shown in Scheme 1. First, the intermediate bis(ether dinitrile) was obtained from the nucleophilic nitro-displacement of 4-nitrophthalonitrile with the phenoxide ions of hydroquinones in DMF. The bis(ether diacid) was prepared by ethanolic potassium hydroxide, and then the generated bis(ether diacid) was cyclodehydrated to yield the final bis(ether anhydride). The structures of the bis(ether anhydride) products were confirmed by elemental analysis, IR and 1H NMR spectra.In the IR spectra, after the cyano group was hydrolyzed to give the carboxyl group, the sharp absorption near 2230 cm−1 characteristic to the cyano group disappeared, and the carbonyl stretching absorption around 1700 cm−1 and the broad O–H absorption in the region between 2550 and 3500 cm−1 appeared. After the bis(ether diacid) was cyclodehydrated to the bis(ether anhydride), the broad O–H absorption disappeared, and the spectrum showed characteristic cyclic anhydride absorbances near 1850 and 1770 cm−1, suggesting the asymmetric and symmetric stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Fig. 1 shows the 1H spectrum of the bis(ether anhydride) monomer 3, in which the proton ortho-positioned from the C
O. Fig. 1 shows the 1H spectrum of the bis(ether anhydride) monomer 3, in which the proton ortho-positioned from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (H1) appeared in the downfield of the spectrum (8.00 ppm) owing to the strong electron-withdrawing effect of the C
O groups (H1) appeared in the downfield of the spectrum (8.00 ppm) owing to the strong electron-withdrawing effect of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, the proton ortho-positioned from the ether group (H4, H5) appeared in the upfield of the spectra (7.28 ppm) owing to the strong electron-donating effect of the –O– group. The corresponding protons on the “phthalic anhydride” units (1,1′;2,2′;3,3′) should appear two slightly different chemical shifts because of different electronic nature.
O group, the proton ortho-positioned from the ether group (H4, H5) appeared in the upfield of the spectra (7.28 ppm) owing to the strong electron-donating effect of the –O– group. The corresponding protons on the “phthalic anhydride” units (1,1′;2,2′;3,3′) should appear two slightly different chemical shifts because of different electronic nature.
Synthesis and characterization of polymers
The PEIs 4a–4e in Scheme 2 were prepared via the conventional two-step method by first reacting equimolar amounts of the dianhydride monomer 3 with diamines monomers (a–d) to form the poly(amic-acid) at ambient temperature, and sequential chemical imidization by adding the mixture of acetic anhydride and triethylamine into a PAA solution at ambient temperature. This was followed by heating at 60 °C to obtain the PEIs 4a–4e.The elemental analysis of the PEIs is listed in Table S1,† and shows that the experimental values are close to the calculated ones. As shown in Table 1, the molecular weights of the resulting PEIs were in the range of 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–172
000–172![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for Mw with the Mw/Mn values of 1.5–1.7, relative to the polystyrene standards. The inherent viscosities were evaluated in DMAc at 25 °C with an Ubbelohde viscometer, and the results fell within the 0.70–0.86 dL g−1 range. The structures of the PEIs were characterized by FTIR (Fig. S3†) and H–H COSY spectra (Fig. 2). The FTIR spectra of PEIs exhibited characteristic imide group absorptions at around 1781 and 1726 cm−1 (typical of imide carbonyl asymmetrical and symmetrical stretch), and did not show an amide carbonyl peak at 1650 cm−1, indicating complete imidization during polycondensation. Also in the spectra there were peaks seen at 1375 cm−1 (C–N stretch), 1090 and 730 cm−1 (imide ring deformation), and some strong absorption bands in the region of 1100–1300 cm−1 which were due to the C–O and C–F stretch.
000 for Mw with the Mw/Mn values of 1.5–1.7, relative to the polystyrene standards. The inherent viscosities were evaluated in DMAc at 25 °C with an Ubbelohde viscometer, and the results fell within the 0.70–0.86 dL g−1 range. The structures of the PEIs were characterized by FTIR (Fig. S3†) and H–H COSY spectra (Fig. 2). The FTIR spectra of PEIs exhibited characteristic imide group absorptions at around 1781 and 1726 cm−1 (typical of imide carbonyl asymmetrical and symmetrical stretch), and did not show an amide carbonyl peak at 1650 cm−1, indicating complete imidization during polycondensation. Also in the spectra there were peaks seen at 1375 cm−1 (C–N stretch), 1090 and 730 cm−1 (imide ring deformation), and some strong absorption bands in the region of 1100–1300 cm−1 which were due to the C–O and C–F stretch.
| Polymer | Inherent viscosity ηinha (dL g−1) | GPC data | DSC | TGA | |||
|---|---|---|---|---|---|---|---|
| Mwb × 104 | Mw/Mnb | Tgc (°C) | T5%d (°C) | T10%d (°C) | Char yielde (%) | ||
| a Determined with 0.5% solutions in a solvent (DMAc) at 25 °C.b Relative to polystyrene standard, using DMF as the eluent.c Baseline shift in the second heating DSC traces, with a heating rate of 20 °C min−1 in nitrogen.d Temperature at 5% and 10% weight loss were recorded by TGA at a heating at 10 °C min−1 in nitrogen.e Residual weight (%) when heated to 800 °C. | |||||||
| PEI-4a | 0.84 | 13.5 | 1.7 | 245 | 591 | 629 | 55 |
| PEI-4b | 0.86 | 16.2 | 1.6 | 228 | 573 | 606 | 56 |
| PEI-4c | 0.85 | 17.2 | 1.6 | 222 | 531 | 575 | 55 |
| PEI-4d | 0.75 | 9.3 | 1.5 | 220 | 507 | 556 | 59 |
| PEI-4e | 0.70 | 8.2 | 1.7 | 208 | 520 | 578 | 53 |
Fig. 2 illustrates the H–H COSY spectra of soluble PEI-4c. Assignments of each proton are also given in these figures, and agree well with the proposed molecular structure. In H–H COSY spectra, the correlated pairs at 7.98/7.50 ppm, 7.87/7.30 ppm, 7.65/7.50 ppm and 7.38/7.13 ppm. The aromatic protons 1 appeared at the most downfield (7.98 ppm) owing to the strong electron-withdrawing effect of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups as a doublet. Accordingly, the connected doublet signal at 7.50 ppm is assigned to the protons 2. The corresponding protons on the “phthalic anhydride” units (1,1′;2,2′;3,3′) should appear two slightly different chemical shifts because of different electronic nature. The aromatic protons 11 appeared at the most upfield (7.13 ppm) owing to the strong electron-donating effect of the –O– groups as a triplet, is also connected with multiple signal at 7.38 ppm arising from protons 10.
O groups as a doublet. Accordingly, the connected doublet signal at 7.50 ppm is assigned to the protons 2. The corresponding protons on the “phthalic anhydride” units (1,1′;2,2′;3,3′) should appear two slightly different chemical shifts because of different electronic nature. The aromatic protons 11 appeared at the most upfield (7.13 ppm) owing to the strong electron-donating effect of the –O– groups as a triplet, is also connected with multiple signal at 7.38 ppm arising from protons 10.
Fig. S4† shows the WAXD patterns of the PEIs 4a–4e. The curves of all the PEIs were broad and without obvious peak features, because of the polymer backbones with multiple trifluoromethyl groups and bulky aromatic side moieties which would increase the disorder of the polymer chains. The amorphous structure endows the obtained PEIs 4a–4e with good solubility.
Solubility of the PEIs
The solubility of synthesized PEIs 4a–4e was tested in various organic solvents and the results are summarized in Table S2.† All the PEIs showed excellent solubility in aprotic polar solvents such as NMP, DMAc and DMSO, and were also soluble in less polar solvents like Py and acetone at room temperature. The excellent solubility of PEIs could be attributed to the presence of flexible ether structure, CF3 and Cl groups, and bulky phenyl substitutes in the dianhydrides, which appeared to force the two phenyl rings into adopting noncoplanar conformation, resulting in inhibition of close packing and reduced interchain interactions to enhance solubility. The PEIs 4d and 4e exhibited better solubility than PEI 4a–4c. PEI-4d and 4e even exhibited excellent solubility in acetone. The good solubility of PEI-4d and 4e was attributed to the high fluorine and ether content in the polymer backbone.Thermal properties of the PEIs
The thermal behavior data of all the PEIs are listed in Table 1. In general, the decreasing order of Tg correlated with both molecular packing and chain conformation (chain rigidity and linearity) of the polymer. The Tg values of PEIs 4a–4e were in the ranges of 208–245 °C, which decreased in the order of 4a > 4b > 4c > 4d > 4e. As we expected, the Tg values of these PEIs depended on the structure of the diamine component and decreased with increasing flexibility of the PEI backbones according to the applied structure of the diamine. PEI-4e derived from 9F–Cl-APB exhibited the lowest Tg because of the presence of two flexible ether units and three –CF3 groups, which would lead to an internal plasticization in addition to the geometry and free volume factors.Thermal stabilities of the PEIs were evaluated by TGA as shown in Fig. 3. All the PEIs had excellent thermal stability, and no obvious decomposition was observed below 500 °C as shown in Table 1. Film samples of PEIs underwent 5% weight losses at 507–591 °C in nitrogen when subjected to TGA with a heating rate of 10 °C min−1. Their char yield at 800 °C in nitrogen was in the range of 53–59 wt%. The TGA data indicated that these PEIs had fairly high thermal stabilities regardless of the introduction of the pendant groups, even though they showed high solubility and optical transparency.
Mechanical and electrical properties of the PEIs
The mechanical properties of the PEI films were summarized in Table 2. The PEI films have tensile strength of 88–120 MPa, tensile modulus of 2.11–2.70 GPa, and elongation at breakage of 15–27%, respectively.| Polymers | Film thickness (μm) | Tensile strength (MPa) | Elongation at break (%) | Youngs' modulus (GPa) | Dielectric constanta | Water uptake (%) |
|---|---|---|---|---|---|---|
| a Dielectric constant of PEIs at 1 MHz. | ||||||
| PEI-4a | 82 | 120 | 15 | 2.70 | 2.80 | 0.52 |
| PEI-4b | 80 | 91 | 25 | 2.41 | 2.75 | 0.50 |
| PEI-4c | 79 | 89 | 27 | 2.25 | 2.73 | 0.43 |
| PEI-4d | 80 | 92 | 20 | 2.20 | 2.69 | 0.32 |
| PEI-4e | 80 | 88 | 18 | 2.11 | 2.77 | 0.30 |
The dielectric constants of the PEI films were evaluated by capacitance method and were also listed in Table 3. All the PEIs showed low k values in the range of 2.69–2.80 at 1 MHz due to the existence of CF3, Cl groups, and bulky phenyl substitutes groups in the main chain, which brought about less efficient chain packing and an increased free volume. As expected, the fluorinated PEI-4e exhibited lower water absorption (0.30%) than PEI-4a (0.52%), PEI-4b (0.50%), PEI-4c (0.43%) and PEI-4d (0.32%), due to the content of hydrophobic CF3 and Cl groups. The low water absorptions ensure that these polymers have stable dielectric performance.
| Polymer | Refractive index | λ0 (nm) | Transmittance at 450 nm (%) | Transmittance at 550 nm (%) | Transmittance at 800 nm (%) | |||
|---|---|---|---|---|---|---|---|---|
| nTEa | nTMb | nAVc | Δnd | |||||
| a In-plane refractive index at 650 nm: nTE.b Out-of-plane refractive index at 650 nm: nTM.c Average refractive index: nAV = (2nTE + nTM)/3.d Birefringence Δn = nTE − nTM. | ||||||||
| PEI-4a | 1.6312 | 1.6181 | 1.6268 | 0.0131 | 369 | 76 | 85 | 87 |
| PEI-4b | 1.6237 | 1.6122 | 1.6186 | 0.0115 | 366 | 80 | 86 | 87 |
| PEI-4c | 1.6092 | 1.5990 | 1.6058 | 0.0102 | 360 | 83 | 87 | 88 |
| PEI-4d | 1.5992 | 1.5901 | 1.5962 | 0.0091 | 358 | 84 | 88 | 90 |
| PEI-4e | 1.6234 | 1.6182 | 1.6217 | 0.0052 | 346 | 88 | 93 | 94 |
Optical properties of the PEIs
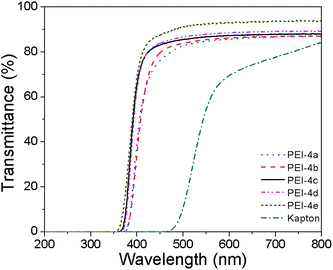
Optical transparency of the PEI films was evaluated by their physical appearance, UV-vis spectra and color intensities. Fig. 4 shows the UV-visible transmittance spectra of the PEI films with thickness of 80 μm, while the cut-off wavelength (absorption edge, λ0) values and the percentages transmittance at different wavelength are listed in Table 3. All the PEIs had shorter λ0 than 370 nm, and exhibited high optical transparency of 76–88% at 450 nm, 85–93% at 550 nm and 87–94% at 800 nm. This contrasted sharply with the typical Kapton film derived PMDA and 4,4′-ODA, which showed a deep yellow color and exhibited λ0 of 455 nm and transmittance at 450 nm of 0% and at 550 nm of 54%. Because of the highly conjugated aromatic structures and intermolecular charge-transfer complex (CTC) formation of PI, most polymers between the UV and the visible area have strong absorption. However, these PEIs which had flexible groups like ether linkages and CF3 groups with bulky side groups in the center of the dianhydride, reduced the intermolecular CTC between alternating electron-donor (diamine) and electron-acceptor (dianhydride) moieties. The PDA, ODA, APB, 6FAPB and 9F–Cl-APB produced nearly colorless PEI films, which could be explained from the decreased intermolecular interactions. Moreover, the CF3 group might weaken the chain-to-chain cohesive force due to a lower polarizability of the C–F bond. For comparison, another photograph of a commercial Kapton film is shown in Fig. 5. The films were photographed against a special background to highlight the transparency. It is easy to see that the PEIs films were much lighter in color than the Kapton film. Wherein, the color of PEI 4c–4e films is close to colorless compared with PEI 4a and 4b. Furthermore, we also investigated the relationship between the transparency of the films and their thickness. According to the data from Fig. 6, with the increase of the thickness of PEI-4d film from 19 to 78 μm, the λ0 increased from 325 to 358 nm. The results indicated that the transparency of the PEI films was heavily dependent on their thickness.The refractive indices of the PEIs 4a–4e were studied using a Gaertner L116B spectroscopic ellipsometer, and the values were in the range of 1.6268–1.5962 at 650 nm, 1.5595–1.6039 at 1310 nm, 1.5541–1.6022 at 1550 nm, respectively, as shown in Fig. 7. In addition, the refractive index decreased with the increase of the wavelength from 600 nm to 1600 nm. In fact, a good linear relationship between the refractive index of the polymer and the feed ratio of the dianhydride (monomer 3 and 6FDA) and diamine (6FAPB and 9F–Cl-APB) was observed as shown in Fig. 8. When the feed ratio of 6FDA was increased from 0 to 100 mol%, the refractive index decreased from 1.5962 to 1.5523 (as shown in Fig. 8a). This was attributed to the fluorinated and chlorinated content of the diamine monomer. On the other hand, we can control the refractive index by controlling the ratio of diamine: when the feed ratio of 6FAPB was increased from 0 to 100 mol%, the refractive index decreased from 1.6217 to 1.5962 (as shown in Fig. 8b). Thus, this method may be better suited for copolymerization for controlling the refractive index in greater scope. A similar chemical structure to ensure that the core and the cladding materials having similar CTE, to prevent cracking between the core and cladding materials due to temperature changes. Since the design and fabrication of waveguide structures depend on the difference in the refractive index between the core and the cladding materials, this linearity of refractive index over such a large range provides tremendous flexibility in the fabrication of waveguide devices using these PEIs.
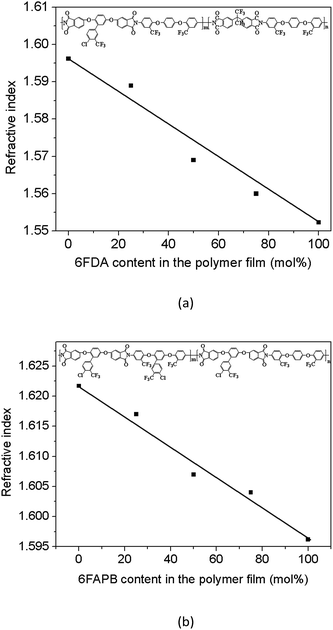 | ||
| Fig. 8 (a) Variation of refractive index at 650 nm with dianhydride 6FDA content. (b) Variation of refractive index at 650 nm with diamine 6FAPB content. | ||
The birefringences (Δn = nTE − nTM) of the PEIs were studied using the prism coupling technique at 650 nm on thin films, and showed low values in the range of 0.0052–0.0131 as shown in Table 3. The fact that nTE values were slightly higher than the nTM ones for all PEIs films reflects the preferential chain orientation parallel to the film plane. It is well known that the birefringence of polymers can be affected by chain flexibility and geometry of the repeat units.35,36 The values of Δn were far lower than conventional aromatic PIs.37 The negligibly small birefringence implied that these polymers with pendant groups had low anisotropy, and the polymer chains were randomly oriented in the film.
It was reported that the replacement of C–H bonds with C–F bonds gave high optical transparency of the polymeric material in the near-infrared (NIR) telecommunication region.38 The propagation losses of the fluorinated PEIs 4a–4e were measured by the NIR absorption spectra, and the spectra are shown in the Fig. 9. In these spectra, the C–H bond vibrational absorption peaks (2νC–H, 1650 nm, and 3δC–H, 1100 nm) and a related peak (2νC–H + δC–H, 1400 nm) can be observed. However, they had small light absorptions at the telecommunication wavelengths of 1310 and 1550 nm due to high fluorine content. The wavelength of 1310 nm or 1550 nm will be used for optical telecommunication, so these PEIs are expected to be applicable to optoelectronic materials.39
Conclusions
A series of novel PEIs (4a–4e) with ether linkages and bulk side groups in the backbone have been successfully synthesized from the novel dianhydride monomer 3 with CF3 and Cl groups and diamines (a–e). It was proved to be a successful process for reducing overall charge transfer complex formation due to either inter- or intramolecular electronic interactions. Thus, the prepared PEIs displayed excellent solubility, high thermal stability, good mechanical properties, and low dielectric constants. It was very interesting that they also had excellent optical properties, including high optical transparency, low birefringence, and low NIR absorptions losses at the communication windows. Moreover, the refractive indices could be controlled precisely by the copolymerization in the range of 1.6217–1.5523 at 650 nm. All the characterizations proved that the resulting PEIs were potential candidates for optical devices and microelectronic applications.Acknowledgements
We thank the National Science Foundation of China (51203172) for the financial support.Notes and references
- M. K. Ghosh and K. L. Mittal, in Polyimides Fundamentals and Applications, Marcel Decker, New York, 1996 Search PubMed.
- D. Wilson, H. D. Stenzenberger and P. M. Hergenrother, in Polyimides, Blackie, Glasgow, London, 1990 Search PubMed.
- W. S. Wong and A. Salleo, in Flexible Electronics Materials and Applications, Springer, New York, 2009 Search PubMed.
- Y. Liu, D. M. Chao and H. Y. Yao, Org. Electron., 2014, 15, 1422–1431 CrossRef CAS.
- H. Lim, W. J. Cho, C. S. Ha, S. Ando, Y. K. Kim and C. H. Park, Adv. Mater., 2002, 14, 1275–1279 CrossRef CAS.
- M. C. Choi, J. C. Hwang, C. Kim, S. Ando and C. S. Ha, J. Polym. Sci., Part A: Polym. Chem., 2001, 48, 1806–1814 CrossRef.
- K. Mizoguchi, Y. Shibasaki and M. Ueda, J. Photopolym. Sci. Technol., 2007, 20, 181–186 CrossRef.
- D. J. Liaw, K. L. Wang, Y. C. Huang, K. R. Lee, J. Y. Lai and C. S. Ha, Prog. Polym. Sci., 2012, 37, 907–974 CrossRef CAS.
- M. Hasegawa and K. Horie, Prog. Polym. Sci., 2001, 26, 259–335 CrossRef CAS.
- J. Yan, Z. Wang, L. Gao and M. Ding, Machromolecules, 2006, 39, 7555–7560 CrossRef CAS.
- Y. T. Chern and J. Y. Tsai, Macromolecules, 2008, 41, 9556–9564 CrossRef CAS.
- D. J. Liaw, F. C. Chang, M. K. Leung, M. Y. Chou and K. Muellen, Macromolecules, 2005, 38, 4024–4029 CrossRef CAS.
- C. P. Yang and Y. Y. Su, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3140–3152 CrossRef CAS.
- K. Kudo, D. Nonokawa, J. Li and S. Shiraishi, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 4038–4044 CrossRef CAS.
- S. H. Hsiao, C. P. Yang, Y. C. Chen, H. M. Wang and W. Guo, J. Appl. Polym. Sci., 2009, 113, 3993–4002 CrossRef CAS.
- C. P. Yang, Y. C. Chen, S. H. Hsiao, W. Guo and H. M. Wang, J. Polym. Res., 2010, 17, 779–788 CrossRef CAS.
- C. P. Yang, S. H. Hsiao and K. H. Chen, Polymer, 2002, 43, 5095–5104 CrossRef CAS.
- C. L. Chung and S. H. Hsiao, Polymer, 2008, 49, 2476–2485 CrossRef CAS.
- G. R. Newkome and C. D. Shreiner, Polymer, 2008, 49, 1–173 CrossRef CAS.
- Z. Qiu, J. Wang, S. Zhang, M. Ding and L. Gao, Polymer, 2006, 47, 8444–8452 CrossRef CAS.
- C. P. Yang, Y. Y. Su, S. J. Wen and S. H. Hsiao, Polymer, 2006, 47, 7021–7033 CrossRef CAS.
- D. M. Knauss and J. B. Edson, Polymer, 2006, 47, 3996–4003 CrossRef CAS.
- C. L. Chung, W. F. Lee, C. H. Lin and S. H. Hsiao, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1756–1770 CrossRef CAS.
- C. Y. Wang, G. Li, X. Y. Zhao and J. M. Jiang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3309–3317 CrossRef CAS.
- Y. T. Chern, J. Y. Tsai and J. J. Wang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2443–2452 CrossRef CAS.
- Y. Xing, D. Wang, H. Gao and Z. H. Jiang, J. Appl. Polym. Sci., 2011, 122, 738–747 CrossRef CAS.
- Y. Liu, Y. H. Zhang, S. W. Guan and Z. H. Jiang, Polymer, 2008, 49, 5439–5445 CrossRef CAS.
- Y. Liu, Y. Xing, Y. H. Zhang, S. W. Guan, H. B. Zhang, Y. Wang, Y. P. Wang and Z. H. Jiang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3281–3289 CrossRef CAS.
- Y. Liu, Y. H. Zhang, S. W. Guan, H. B. Zhang, X. G. Yue and Z. H. Jiang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6269–6279 CrossRef CAS.
- Y. H. Zhang, H. B. Zhang, J. X. Mu, Z. H. Jiang and S. W. Guan, J. Appl. Polym. Sci., 2013, 127, 1834–1841 CrossRef.
- W. Groh, Makromol. Chem., 1988, 189, 2861–2874 CrossRef CAS.
- K. Han, H. J. Lee and T. H. Rhee, J. Appl. Polym. Sci., 1999, 74, 107–112 CrossRef CAS.
- B. J. Liu, W. Hu, Y. H. Jin, C. H. Chen, Z. H. Jiang, W. J. Zhang, Z. W. Wu and T. Matsumobo, Polymer, 2004, 45, 3241–3247 CrossRef CAS.
- K. Xie, S. Y. Zhang, J. G. Liu, M. H. He and S. Y. Yang, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 2581–2590 CrossRef CAS.
- H. Ma, A. K.-Y. Jen and L. R. Dalton, Adv. Mater., 2002, 14, 1339–1365 CrossRef CAS.
- T. Kaino, J. Polym. Sci., Part A: Polym. Chem., 1987, 25, 37–46 CrossRef CAS.
- Y. M. Kim, E. Lim, I.-N. Kang, B.-J. Jung, J. Lee, B. W. Koo, L.-M. Do and H.-K. Shim, Macromolecules, 2006, 39, 4081–4085 CrossRef CAS.
- C. T. Yen and W. C. Chen, Macromolecules, 2003, 36, 3315–3319 CrossRef CAS.
- T. Matsuura, S. Ando, S. Sasaki and F. Yamamoto, Macromolecules, 1994, 27, 6665–6670 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19770h |
| This journal is © The Royal Society of Chemistry 2016 |