Nonlinear optical effects in nitrogen-doped graphene
Abstract
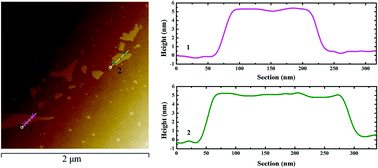
The band structure of graphene can be adjusted by incorporating other elements or functional groups, correspondingly, the response to an external light field will also be changed. In this study, we investigated the nonlinear optical (NLO) responses of N-doped graphene nanosheets (N-Gns) with an open aperture Z-scan method, under the excitation of picosecond laser pulses. To the best of our knowledge, this is the first time that two-photon absorption (2PA) of N-Gns has been reported. The normalized nonlinear transmission demonstrates that with the increasing excitation energy, the saturable absorption (SA) and the 2PA appear successively. Compared to the 532 nm excitation, the 1064 nm excitation exhibits smaller saturable intensity Is and larger 2PA coefficient β. At the same time, the N-Gns present superior NLO property compared to pure graphene. Consequently, N-Gns will be a promising candidate for SA or optical limiting (OL) applications.

 Please wait while we load your content...
Please wait while we load your content...