Bifunctional monomer molecularly imprinted polymers based on the surface of multiwalled carbon nanotubes for solid-phase extraction of tartrazine from drinks
Lingmin Wu,
Fei Liu,
Gailing Wang,
Zhian Guo and
Jingchan Zhao*
Key Laboratory of Synthetic and Natural Functional Molecular Chemistry of Ministry of Education, College of Chemistry & Material Science, Northwest University, Xi’an, 710127, China. E-mail: zhaojc@nwu.edu.cn; Fax: +86-029-81535026; Tel: +86-029-81535023
First published on 8th December 2015
Abstract
A novel composite material consisting of multi-walled carbon nanotube (MWNTs)-molecularly imprinted polymers (MIPs) for a tartrazine assay was synthesized by a surface molecular imprinting technique. The molecularly imprinted polymers were prepared with beta-cyclodextrins and [2-(methacryloyloxy) ethyl]trimethylammonium chloride (DMC) as bifunctional monomers. The MWNTs-MIPs were characterized using transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FT-IR). Adsorption experiments showed that the MWNTs-MIPs exhibited a high capacity and favourable selectivity toward tartrazine, and the maximum adsorption capacity, qm, and distribution coefficient, kd, were estimated to be 135.15 mg g−1 and 14.72, respectively. A SPE-HPLC analytical method was developed and used to assay tartrazine in five drinks. The recoveries were in the range from 88.6–102.4% with a relative standard deviation below 5.0%. The tartrazine content was lower in all samples than the permitted maximum limit set by the European Commission and Ministry of Health of the People’s Republic of China. A t test demonstrated that this method had no significant difference to the Chinese standard method of GB/T 5009.35-2003 at P = 0.95.
1. Introduction
Recently, molecularly imprinted polymers (MIPs) have attracted tremendous interest owing to their distinct advantages, including a predetermined recognition ability, mechanical and chemical stability, durability, reusability and lower preparation costs.1 MIPs can act as sorbents, and are usually used in solid-phase extraction (SPE) for separations and enrichments.2–4 Conventional bulk MIPs exhibit low-affinity binding and low-rate mass transfer, and to overcome these disadvantages, surface molecular imprinting techniques have been developed. The advantages of surface molecular imprinting include improving mass transfer, increasing affinity binding and decreasing the high diffusion barrier of the template by fixing MIPs on a support substrate.5–7 To date, various materials, such as magnetic nanoparticles,8,9 hydroxyapatite,10 stainless steel fiber,11 silica gel,12 and TiO2 have been used as the support matrixes for the preparation of MIPs.13 Multi-walled carbon nanotubes (MWNTs) with unique mechanical properties and extremely large surface areas can be an excellent candidate as an available support material due to the binding sites in the outer layer of the composite, which will improve the accessibility of the template molecule and reduce the binding time.14–16 In order to improve the weak interfacial adhesion, covalent and noncovalent functionalization of MWNTs is commonly used. Vinyl groups and amine groups are selected to link to the surface of the MWNTs by covalent bonding resulting in strong interfacial interactions between the MWNTs and MIPs.17In the process of MIP synthesis, in order to strengthen the multi-noncovalent interactions between the template and functional monomers, bifunctional MIPs are the best choice due to their high adsorption capacity and selectivity. Xu and co-workers synthesized parathion-methyl-templated MIPs using methacrylamide and 4-vinyl pyridine as bifunctional monomers, and the MIP was successfully used to detect pesticide in the soil.18 Zhang and co-workers adopted acryloyl-β-cyclodextrin and methacrylic acid as stimuli-recognition elements towards erythromycin imprinting.19 In our previous work, we had synthesized a novel bifunctional MIP using beta-cyclodextrins–maleic anhydride and [2-(methacryloyloxy)ethyl]trimethylammonium chloride as co-functional monomers and utilized it as a sorbent in SPE to extract congo red in food.20
Synthetic colorants are often added to food products, and most contain azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) functional groups and aromatic ring structures which are harmful to human health.21 Tartrazine is one of the most common azo colorants, and high performance liquid chromatography (HPLC) is the most frequently used method in its determination.22 However, an enrichment procedure by molecularly imprinted solid-phase extraction (MISPE) is usually essential, especially for food sample analysis by the HPLC method.23
N) functional groups and aromatic ring structures which are harmful to human health.21 Tartrazine is one of the most common azo colorants, and high performance liquid chromatography (HPLC) is the most frequently used method in its determination.22 However, an enrichment procedure by molecularly imprinted solid-phase extraction (MISPE) is usually essential, especially for food sample analysis by the HPLC method.23
In this paper, a novel MIP with an improved selectivity for tartrazine on the surface of MWNTs was synthesised. Beta-cyclodextrins (β-CD) and [2-(methacryloyloxy) ethyl]trimethylammonium chloride (DMC) were chosen as co-functionals, and the performance of the MIPs utilized as solid phase extraction sorbents was investigated in detail. The results demonstrated that the MWNTs-MIPs could selectively recognize tartrazine. Combining SPE with HPLC techniques, the MWNTs-MIPs were used for selective preconcentration of tartrazine from five drink samples successfully.
2. Materials and methods
2.1. Reagent and chemicals
Hydroxyl multi-wall carbon nanotubes (95%, outer diameters ≤ 8 nm, lengths ranging from 10 μm to 30 μm) were purchased from Chengdu institute of organic chemistry, Chinese academy of sciences. Tartrazine, carmine and sunset yellow were supplied by Pure Crystal Shanghai Reagent Co. Ltd. (Shanghai, China). Their chemical structures are shown in Fig. 1. DMC, N,N-methylenebisacryl amide (MBA), methyl methyl acrylate (MMA), dimethylformamide (DMF) and ammonium persulfate (APS) were obtained from Gaoyu Special Chemical Co. Ltd (Tianjin, China). Beta-cyclodextrin sodium (β-CD−Na+), and γ-(2,3-epoxypropoxy)propyltrimethoxysilane (KH-560) were bought from Nanjing Shuguang Chemical Group Co, Ltd. (Jiangsu, China). Methanol, acetic acid, ammonia solution (28.0%) and ammonium acetate were obtained from Guangzhou Chemical Reagent Factory (Guangzhou, China). HPLC-grade methanol and water were purchased from Burdick & Jackson (USA) and Hangzhou Wahaha Group (Zhejiang, China), respectively.2.2. Instruments
A Tecnai G2 F20 S-TWIN microscope was used to obtain transmission electron microscopy (TEM) images (USA). The solid phase extraction experiment was conducted with an ASE-12 solid phase extraction system from Automatic Science Instrument Co. Ltd (Tianjin, China). The spectra were obtained using a Shimadzu UV-2550 spectrophotometer. 10.0 μL of solution was analyzed using an Agilent 1100 HPLC system (USA). The separations were carried out using a Germil-p C18 column (150 mm × 4.6 mm, 5 μm) from Wu-Ben Biotechnology Co. Ltd (Xi’an, China). Isocratic elution was carried out at a flow rate of 1.0 mL min−1, with a methanol–0.02 M ammonium acetate buffer solution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) as the mobile phase which was filtered through a 0.45 μm membrane filter prior to use. The column oven temperature was set at 30 °C. The detection of analytes was carried out with the photodiode-array UC detector at 254 nm.
90, v/v) as the mobile phase which was filtered through a 0.45 μm membrane filter prior to use. The column oven temperature was set at 30 °C. The detection of analytes was carried out with the photodiode-array UC detector at 254 nm.
2.3. Preparation of MWNTs grafted epoxy (MWNTs-epoxy)
Hydroxyl MWNTs (4.00 g) were dispersed in 30.0 mL of DMF and sonicated for 30 min, 1.0 mL of acetic acid was added slowly to the solution under vigorous stirring and the mixture was heated to 90 °C, then KH-560 solution (2.8 mL KH-560 in 20.0 mL DMF) was added drop-wise under mechanical stirring and nitrogen protection. The reaction mixture was stirred for 12 h to obtain epoxy group functionalized MWNTs.2.4. Synthesis of MWNTs-methyl methyl acrylate/beta-cyclodextrins (MWNTs-MMA/β-CD)
β-CD−Na+ (4.80 g) was transferred into MWNT-epoxy solution and reacted at 90 °C for 12 h. Then 1.8 mL of MMA and 80 μL of BF3·Et2O were added to the mixture, which was incubated for 12 h under stirring for prepolymerization at 45 °C. A black precipitate obtained was filtrated and washed several times with DMF, methanol, and acetone, in order, and the obtained MWNTs-MMA/β-CD was dried under vacuum at 30 °C for preparation of the MWNTs-MIPs.2.5. Preparation of tartrazine imprinted polymers (MWNTs-MIPs)
MWNTs-MMA/β-CD (4.00 g), tartrazine (0.25 g) and 50.0 mL of 0.05 M sodium phosphate buffer of pH = 7.0 were mixed under sonication for 0.5 h and stirred for 24 h at 60 °C. Then 120 μL of DMC was added to the previous pre-polymerization mixture, and after the process of 4 h of self-assembly, APS (0.05 g) and MBA (0.75 g) were added and the mixture was purged with nitrogen to remove oxygen for 30 min. Next, the flask was sealed and left polymerizing at 60 °C for 24 h. The resulting particles were washed using methanol–ammonia (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) solution in a Soxhlet extraction system until no tartrazine could be detected by HPLC. For comparison, non-imprinted polymers (NIPs) were prepared using the same procedure, only without using the template molecule in the polymerization process.
3, v/v) solution in a Soxhlet extraction system until no tartrazine could be detected by HPLC. For comparison, non-imprinted polymers (NIPs) were prepared using the same procedure, only without using the template molecule in the polymerization process.
2.6. Preparation of SPE column
The SPE columns dry-packed with 100.0 mg of the MWNTs-MIPs or MWNTs-NIPs were leached with methanol (10 mL) and water (10 mL), in order, and stored in a vacuum oven. Prior to loading the sample, the column was preconditioned with 5.0 mL of methanol and 5.0 mL of water in succession.2.7. Adsorption study
An amount of 50.0 mg of the MWNTs-MIPs, MWNTs-NIPs or raw MWNTs was suspended in 25.0 mL of tartrazine solutions with initial concentrations of tartrazine ranging from 0.1 to 6.0 mmol L−1 for 3 h at 25 °C and the tubes were sealed and oscillated at regular time intervals. The mixture was centrifuged and the concentration of free tartrazine in the supernate was measured using HPLC.Competitive adsorption of tartrazine and its structurally similar compounds carmine and sunset yellow from their mixture was investigated in a batch system. A solution (25.0 mL) containing 6.0 mmol L−1 tartrazine, carmine and sunset yellow was adsorbed with MWNTs-MIPs, MWNTs-NIPs and raw MWNTs (50.0 mg) at 25 °C for 3 h and then centrifuged, and the concentrations of tartrazine, carmine and sunset yellow were determined by HPLC.
2.8. Sample pretreatment
Five drinks products including different types of canned soft drinks were purchased from several supermarkets in Xian City. Samples were degassed by sonication for 30 min, and centrifuged for 15 min at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to separate insoluble substances and the supernatant was mixed as loading solution. For the spiking experiments, an analyte standard was added. For the recovery test, a tartrazine standard was added to the samples.
000 rpm to separate insoluble substances and the supernatant was mixed as loading solution. For the spiking experiments, an analyte standard was added. For the recovery test, a tartrazine standard was added to the samples.
3. Results and discussion
3.1. Characterization of the MWNTs-MIPs
FT-IR spectroscopy was used to detect the surface chemical functional groups at different processing stages. The FT-IR spectra of the MWNTs-MIPs, MWNTs-MMA/β-CD and raw MWNTs are shown in Fig. 2. The broad peak at the range of 3200–3700 cm−1 in spectra a, b and c was related to the stretching vibrations of O–H. The absorbance band at 1564 cm−1 in spectrum c corresponded to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and the peaks at 1195 cm−1 resulted from the C–O vibration of the structure of the hydroxyl MWNTs. In contrast to spectrum c, the absorbance peaks at 1037, 1656 and 2920 cm−1 in spectrum b were attributed to the stretching vibrations of Si–O–Si, C
C bonds and the peaks at 1195 cm−1 resulted from the C–O vibration of the structure of the hydroxyl MWNTs. In contrast to spectrum c, the absorbance peaks at 1037, 1656 and 2920 cm−1 in spectrum b were attributed to the stretching vibrations of Si–O–Si, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–H, respectively. The absorbance intensities of the peaks at 3363 cm−1 were strengthened due to the structure of β-CD, and these confirmed the successful coating of the MMA/β-CD layer on the MWNTs. Compared with spectrum b, the absorbance intensities of the peaks at 1564 cm−1 in spectrum a were weakened, which was attributed to the breaking of the C
O, and C–H, respectively. The absorbance intensities of the peaks at 3363 cm−1 were strengthened due to the structure of β-CD, and these confirmed the successful coating of the MMA/β-CD layer on the MWNTs. Compared with spectrum b, the absorbance intensities of the peaks at 1564 cm−1 in spectrum a were weakened, which was attributed to the breaking of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. New absorption bands which appeared at 1454 cm−1 were the CH2–N bond and the stretching vibration of C
C bond. New absorption bands which appeared at 1454 cm−1 were the CH2–N bond and the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1718 cm−1) revealed the existence of MBA. All these results demonstrated that the tartrazine MIPs had been grafted onto the MWNTs. The synthetic route of introducing the silicon–oxygen group and further grafting MIPs onto the MWNT surface is illustrated in Fig. 3.
O (1718 cm−1) revealed the existence of MBA. All these results demonstrated that the tartrazine MIPs had been grafted onto the MWNTs. The synthetic route of introducing the silicon–oxygen group and further grafting MIPs onto the MWNT surface is illustrated in Fig. 3.
TEM images of the raw MWNTs, and MWNTs-MIPs are shown in Fig. 4a and b. The average diameter of the MWNTs-MIPs composite is larger than that of the raw MWNTs. In addition, the MWNTs-MIPs exhibited a slightly rough surface compared to the pristine raw MWNTs, which may assist tartrazine to be rebound rapidly. The result indicated that the MIP layer was modified onto the surface of the MWNTs.
3.2. Adsorption properties
It can be seen from Fig. 5 that the adsorption capacities of all the absorbents increased with increasing tartrazine concentration. As for the MWNTs-MIPs and raw MWNTs, their maximum binding capacities were slightly different. The maximum binding capacity of the MWNTs-MIPs was higher than that of the MWNTs-NIPs. The Freundlich isotherm model and Langmuir isotherm model were used to estimate the binding properties of the MIPs.
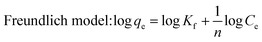 | (1) |
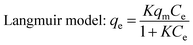 | (2) |
 | ||
| Fig. 5 Adsorption isotherm curves of raw MWNTs, MWNTs-MIPs and MWNTs-NIPs toward tartrazine. Amount of polymers, 50.0 mg; volume, 25.0 mL; initial concentration of tartrazine, 0.1 mmol L−1. | ||
According to the Langmuir model, adsorption occurred uniformly on the active spots of the adsorbent. Once a template molecule occupied the site, no further adsorption could take place at this site. The Freundlich model is a multilayer adsorption model and the surface of the adsorbent is not uniform. The fitting relation coefficients of the MWNTs-MIPs and MWNTs-NIPs are given in Table 1. The statistics show that the absorption properties of the MWNTs-MIPs and MWNTs-NIPs are better for the Langmuir model and the value of R2 was 0.9950 and 0.9960, respectively. From the linear regression equation of the MWNTs-MIPs, the values of K and qm were 0.043 and 135.15 mg g−1, respectively. The adsorption process of tartrazine onto tartrazine-MIP could be considered a monolayer adsorption.
| The type of polymer | Freundlich model | Kf | R2 | Langmuir model | K | R2 |
|---|---|---|---|---|---|---|
| MWNTs-MIPs | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = 0.32 qe = 0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce ± 1.19 Ce ± 1.19 |
1.19 | 0.8223 | 1/qe = 0.17/Ce ± 0.0074 | 0.043 | 0.9950 |
| MWNTs-NIPs | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = 0.24 qe = 0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce ± 1.41 Ce ± 1.41 |
1.41 | 0.8254 | 1/qe = 0.20/Ce ± 0.0088 | 0.044 | 0.9960 |
3.3. Specific recognition of MWNTs-MIPs
Competitive adsorption studies were performed with tartrazine, sunset yellow and carmine. The selectivity of these materials could be frequently evaluated by the distribution coefficient (kd), selectivity coefficient (k), and relative selectivity coefficient (k′) obtained from the competitive binding experiments.24 ,
, 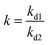 , and
, and  , where Ci and Cf were the initial and final concentration, respectively. The results are listed in Table 2. The kd of tartrazine in the MWNTs-MIPs was 14.72, which was four times that of sunset yellow and carmine, and these data demonstrate that the MWNTs-MIPs have a high recognition toward tartrazine in comparison with sunset yellow and carmine. The kd of tartrazine, sunset yellow, and carmine in the MWNTs-NIPs or raw MWNTs was almost the same, probably because after the polymerization of no template, crosslinking agent and functional monomers, a dense and less porous NIP film on the MWNT surface emerged, and this NIP layer did not improve the specific surface area and the pore size distributions of the MWNTs. Consequently, they did not exhibit obvious differences in their rebinding capacities toward tartrazine, sunset yellow and carmine, which demonstrated their nonspecific adsorption.
, where Ci and Cf were the initial and final concentration, respectively. The results are listed in Table 2. The kd of tartrazine in the MWNTs-MIPs was 14.72, which was four times that of sunset yellow and carmine, and these data demonstrate that the MWNTs-MIPs have a high recognition toward tartrazine in comparison with sunset yellow and carmine. The kd of tartrazine, sunset yellow, and carmine in the MWNTs-NIPs or raw MWNTs was almost the same, probably because after the polymerization of no template, crosslinking agent and functional monomers, a dense and less porous NIP film on the MWNT surface emerged, and this NIP layer did not improve the specific surface area and the pore size distributions of the MWNTs. Consequently, they did not exhibit obvious differences in their rebinding capacities toward tartrazine, sunset yellow and carmine, which demonstrated their nonspecific adsorption.
| Parameter | Adsorbent | MWNTs-MIPs | MWNTs-NIPs | Raw MWNTs |
|---|---|---|---|---|
| The adsorption capacity (mg g−1) | Tartrazine | 85.44 ± 2.37 | 27.77 ± 1.32 | 29.19 ± 1.75 |
| Sunset yellow | 21.26 ± 1.25 | 22.17 ± 1.67 | 39.01 ± 2.09 | |
| Carmine | 15.62 ± 0.84 | 34.62 ± 1.96 | 31.35 ± 2.18 | |
| Kd | Tartrazine | 14.72 ± 0.29 | 4.78 ± 0.15 | 5.02 ± 0.18 |
| Sunset yellow | 3.38 ± 0.18 | 3.52 ± 0.20 | 6.20 ± 0.20 | |
| Carmine | 3.42 ± 0.13 | 7.58 ± 0.23 | 6.86 ± 0.24 | |
| K | Sunset yellow | 4.35 ± 0.09 | 1.36 ± 0.03 | 0.81 ± 0.02 |
| Carmine | 4.29 ± 0.09 | 0.63 ± 0.02 | 0.73 ± 0.03 | |
| K′ | Sunset yellow | — | 3.20 ± 0.08 | 5.37 ± 0.11 |
| Carmine | — | 6.81 ± 0.11 | 5.88 ± 0.12 |
3.4. Optimization of MWNTs-MIPs/SPE
Successful selective adsorption due to hydrophobic interactions and steric complementarity to the analytes is possible in aqueous solutions by optimizing washing, and elution conditions. In order to evaluate the effect of the washing solvent in the MWNTs-MIPs/SPE procedure, and considering that tartrazine has good polarity such that it is easy to dissolve in water, 10.0 mL of different washing solvents involving methanol/water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 3
5, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1
7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) were investigated. The experimental results showed that excess methanol could result in the loss of tartrazine, and in view of the recoveries obtained and the ability to remove the matrix of polar compounds, the ratio of methanol and water at 1
9, v/v) were investigated. The experimental results showed that excess methanol could result in the loss of tartrazine, and in view of the recoveries obtained and the ability to remove the matrix of polar compounds, the ratio of methanol and water at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 is the optimized composition of the washing solution.
9 is the optimized composition of the washing solution.
5.0 mL of methanol containing 10–70% ammonia was used to elute tartrazine from the MWNTs-MIPs/SPE column. As shown in Fig. 6, 5.0 mL of the methanol/ammonia (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) mixture solution was employed as the eluting solution which could elute the most tartrazine.
3, v/v) mixture solution was employed as the eluting solution which could elute the most tartrazine.
 | ||
Fig. 6 The recovery of tartrazine in different methanol/ammonia ratios of a 5 mL elution solution (methanol/ammonia, v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 7 1; 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 5 3; 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; 3 5; 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). 7). | ||
3.5. Repeatability testing of MWNTs-MIPs-SPE
The regenerated SPE was assessed for rebinding the templates (tartrazine). According to the optimized MIP-SPE procedure, tartrazine standard solutions (20.00 mg L−1) were passed through the same MWNTs-MIPs-SPE column at different time points seven times interday or interweek, and the tartrazine recoveries in Table 3 display that the binding capacity for tartrazine maintained a high recovery of over 96.0% within 1 day or 1 week, which illustrated that the recognized sites were stable and the material could be reusable after a regeneration process. Few studies about the reusability of MWNTs-MIPs-SPE have been reported, so the characteristics of the sorbents reported here are superior to other MIP materials.| Item | Spiked level (mg L−1) | Found level (mg L−1) | Recoveries (%) | RSD (%) (n = 7) |
|---|---|---|---|---|
| Inter-day | 20.00 | 19.75 | 98.8 | 1.9 |
| Inter-week | 20.00 | 19.20 | 96.0 | 3.2 |
3.6. Application for real sample analysis
| Drink type | Mean level (mg kg−1) | Amount of added (mg kg−1) | Amount of found (mg kg−1) | Recovery (%) | RSD (n = 3) (%) |
|---|---|---|---|---|---|
| Drink 1 | 16.4 | 50.0 | 67.6 ± 2.4 | 102.4 | 3.5 |
| Drink 2 | 11.5 | 50.0 | 60.3 ± 0.6 | 97.5 | 1.0 |
| Drink 3 | 6.1 | 50.0 | 51.9 ± 2.4 | 91.7 | 4.6 |
| Drink 4 | 39.2 | 50.0 | 83.5 ± 1.9 | 88.6 | 2.3 |
| Drink 5 | 2.5 | 50.0 | 50.0 ± 0.5 | 95.1 | 1.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S/N = 3), and with an executed volume of 50.0 mL. Also, the quantification limit (QL) was calculated at a peak area when the peak height/noise ratio was 10
1 (S/N = 3), and with an executed volume of 50.0 mL. Also, the quantification limit (QL) was calculated at a peak area when the peak height/noise ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The DL and QL correspond to 8.33 × 10−5 and 2.78 × 10−5 ng L−1, respectively. And the RSDs were ranged from 2.9–5.6%. At the same time, the five drink samples were determined by the Chinese standard method GB/T 5009.35-2003 to verify this method. The results of the comparison are presented in Table 5. The results displayed that the t values were in range of 0.28–1.86, when P = 0.95 and f = 5, t0.05,5 = 2.58, t < t0.05,5, so the method has no significant difference with the Chinese standard method. In China and the European Union, the permitted maximum limit of tartrazine additive in a drink is 0.1 g kg−1 (GB2760-2011, Directive of the European Parliament and of the council 94/36/EC−1),25 and the content of tartrazine in these five drinks is under this level.
1. The DL and QL correspond to 8.33 × 10−5 and 2.78 × 10−5 ng L−1, respectively. And the RSDs were ranged from 2.9–5.6%. At the same time, the five drink samples were determined by the Chinese standard method GB/T 5009.35-2003 to verify this method. The results of the comparison are presented in Table 5. The results displayed that the t values were in range of 0.28–1.86, when P = 0.95 and f = 5, t0.05,5 = 2.58, t < t0.05,5, so the method has no significant difference with the Chinese standard method. In China and the European Union, the permitted maximum limit of tartrazine additive in a drink is 0.1 g kg−1 (GB2760-2011, Directive of the European Parliament and of the council 94/36/EC−1),25 and the content of tartrazine in these five drinks is under this level.
| Drink type | The measured value (mg kg−1) | GB measured value (mg kg−1) | F | S | t Test value |
|---|---|---|---|---|---|
| Drink 1 | 16.42 ± 2.1 | 17.26 ± 1.0 | 4.41 | 1.0 | 1.46 |
| Drink 2 | 11.55 ± 1.1 | 12.14 ± 0.6 | 3.20 | 0.6 | 1.70 |
| Drink 3 | 6.11 ± 0.9 | 6.97 ± 0.8 | 1.26 | 0.8 | 1.86 |
| Drink 4 | 39.15 ± 1.8 | 38.39 ± 1.5 | 2.25 | 1.5 | 0.28 |
| Drink 5 | 2.49 ± 0.5 | 2.71 ± 0.3 | 2.28 | 0.3 | 1.26 |
4. Conclusions
In this study, a composite material based on MWNTs polymerized with a layer of bifunctional monomers of molecularly imprinted tartrazine was synthesized. The resultant MWNTs-MIPs possess some attractive characteristics, such as great levels of adsorption and good selectivity. The imprinted material was applied as a sorbent for the enrichment and determination of tartrazine in real drink samples by SPE-HPLC. It exhibited excellent extraction characteristics for tartrazine. The MIP grafting method also provides a significant reference for other radical polymerization reactions based on MWNTs.Acknowledgements
This work was financially supported by the Shaanxi Provincial Science and Development Projects (2012k08-14).References
- M. Karimi, F. Aboufazeli, H. Zhad, O. Sadeghi and E. Najafi, Food Analytical Methods, 2014, 7, 73–80 CrossRef
.
- J. Yu, S. Wang, G. Zhao, B. Wang, L. Ding, X. Zhang, J. Xie and F. Xie, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 958, 130–135 CrossRef CAS PubMed
.
- J. Zhao, B. Hana, Y. Zhang and D. Wang, Anal. Chim. Acta, 2007, 603, 87–92 CrossRef CAS PubMed
.
- S. Zhong, Y. Kong, L. Zhou, C. Zhou, X. Zhang and Y. Wang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 945–946, 39–45 CrossRef CAS PubMed
.
- D. Kryscio and N. Peppas, Anal. Chim. Acta, 2012, 718, 109–115 CrossRef CAS PubMed
.
- J. Liu, K. Yang, Q. Deng, Q. Li, L. Zhang and Z. Liang, Chem. Commun., 2011, 47, 3969–3971 RSC
.
- W. Zhang, L. Qin, X. He, W. Li and Y. Zhang, J. Chromatogr. A, 2009, 1216, 4560–4567 CrossRef CAS PubMed
.
- Q. Gai, F. Qu, Z. Liu, R. Dai and Y. Zhang, J. Chromatogr. A, 2010, 1217, 5035–5042 CrossRef CAS PubMed
.
- M. Zhang, X. Zhang, X. He, L. Chen and Y. Zhang, Nanoscale, 2012, 4, 3141–3147 RSC
.
- B. Zhang, J. Zhao, B. Sha and M. Xian, Anal. Methods, 2012, 4, 3187–3192 RSC
.
- X. Hu, G. Dai, J. Huang, T. Ye, H. Fan, T. Youwen, Y. Yu and Y. Liang, J. Chromatogr. A, 2010, 1217, 5875–5882 CrossRef CAS PubMed
.
- W. Cheng, Z. Liu and Y. Wang, Talanta, 2013, 116, 396–402 CrossRef CAS PubMed
.
- W. Zhang, Y. Li, Q. Wang, C. Wang, P. Wang and K. Mao, Environ. Sci. Pollut. Res., 2013, 20, 1431–1440 CrossRef CAS PubMed
.
- E. Lee, D. Park, D. Lee and B. Kim, Colloids Surf., A, 2008, 313–314, 202–206 Search PubMed
.
- F. Tan, M. Deng, X. Liu, H. Zhao, X. Li, X. Quan and J. Chen, J. Sep. Sci., 2011, 34, 707–715 CrossRef CAS PubMed
.
- D. Zhang, D. Yu, W. Zhao, Q. Yang, H. Kajiura, Y. Li, T. Zhou and G. Shi, Analyst, 2012, 137, 2629–2636 RSC
.
- X. Chen, Z. Zhang, X. Yang, J. Li, Y. Liu, H. Chen, W. Rao and S. Yao, Talanta, 2012, 99, 959–965 CrossRef CAS PubMed
.
- S. Xu, C. Guo and Y. Li, J. Hazard. Mater., 2014, 264, 34–41 CrossRef CAS PubMed
.
- Z. Zhang, X. Yang, H. Zhang, M. Zhang, L. Luo, Y. Hu and S. Yao, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1617–1624 CrossRef CAS PubMed
.
- F. Liu, S. Zhang, G. Wang, J. Zhao and Z. Guo, RSC Adv., 2015, 5, 22811–22817 RSC
.
- K. Amin, H. Hameid and A. Elsttar, Food Chem. Toxicol., 2010, 48, 2994–2999 CrossRef CAS PubMed
.
- S. Bonan, G. Fedrizzi, S. Menotta and C. Elisabetta, Dyes Pigm., 2013, 99, 36–40 CrossRef CAS
.
- M. Sanagi, S. Salleh, W. Ibrahim, A. Naim, D. Hermawan, M. Miskama, I. Hussain and Y. Aboul-Enein, J. Food Compos. Anal., 2013, 32, 155–161 CrossRef CAS
.
- G. Fang, J. Feng, Y. Yan, C. Liu and S. Wang, Food Analytical Methods, 2014, 7, 345–351 CrossRef
.
- EC, 1994, Directive of the European Parliament and of the council 94/36/EC of June 30, 1994 on colours for use in food stuffs, Official J. L237, 13, 10/9/1994.
| This journal is © The Royal Society of Chemistry 2016 |





